Benzylidene-bis-(4-Hydroxycoumarin) and Benzopyrano-Coumarin Derivatives: Synthesis, 1H/13C-NMR Conformational and X-ray Crystal Structure Studies and In Vitro Antiviral Activity Evaluations
Abstract
:1. Introduction
2. Results and Discussion
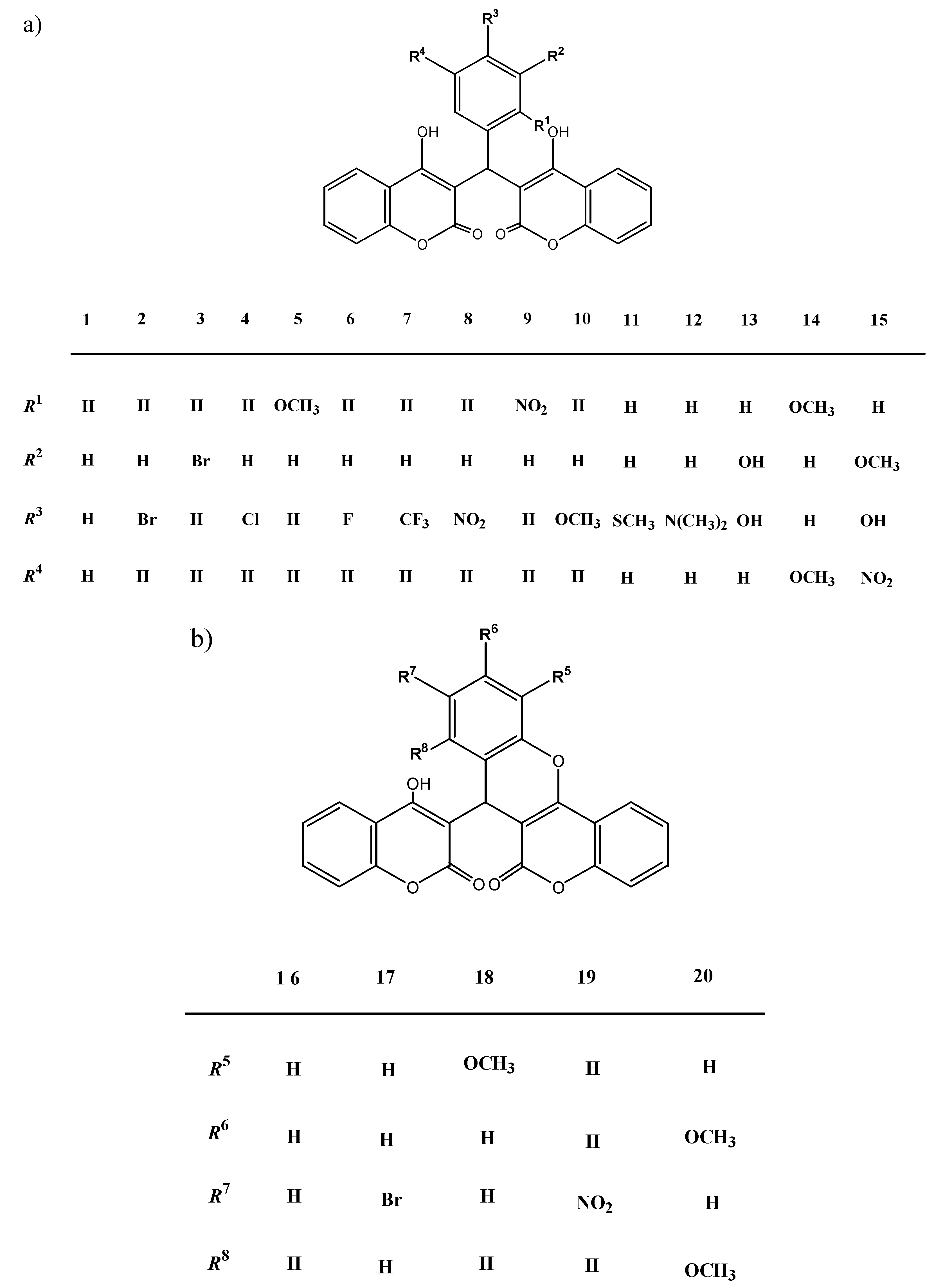
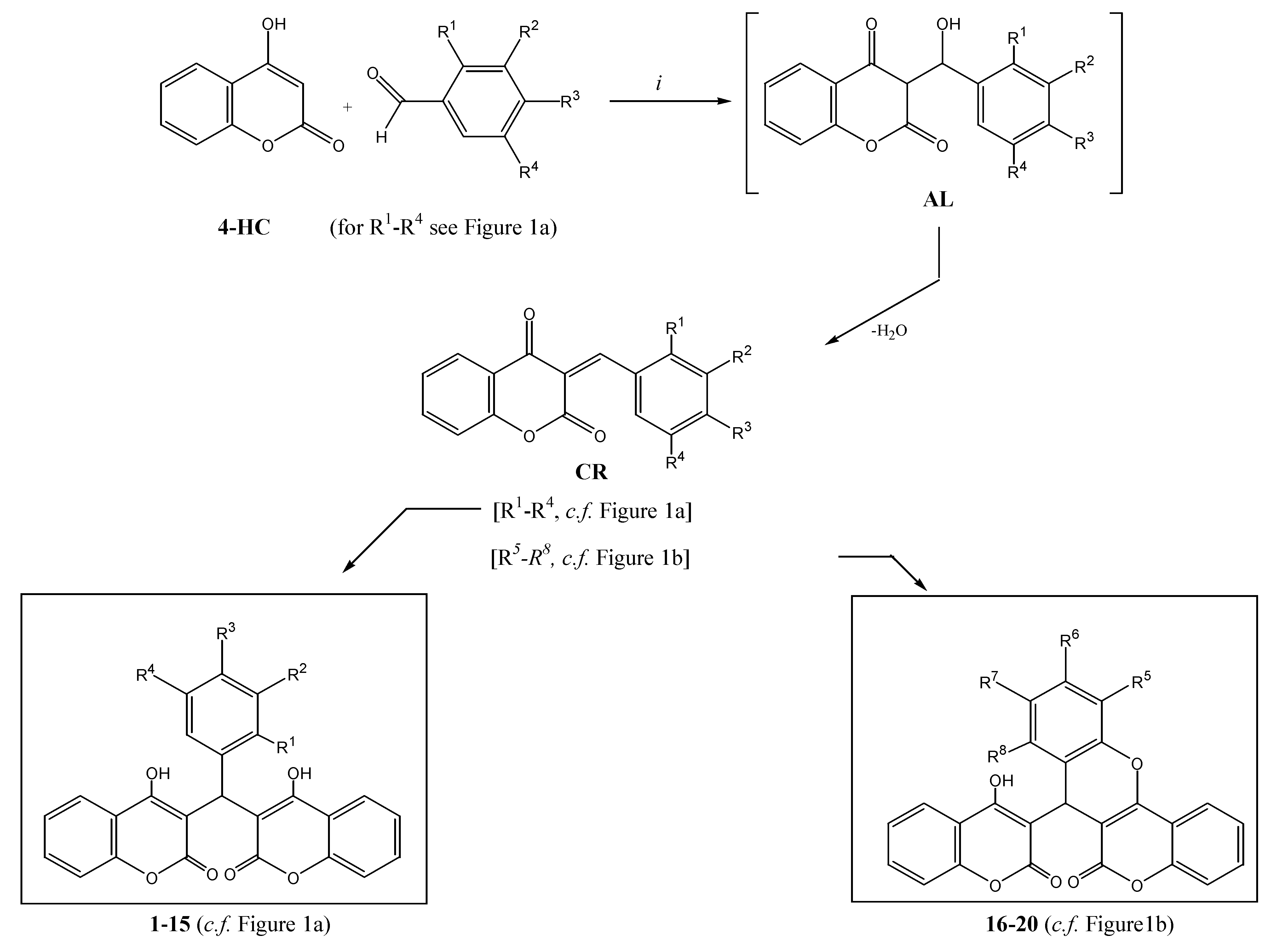
2.1. Chemistry
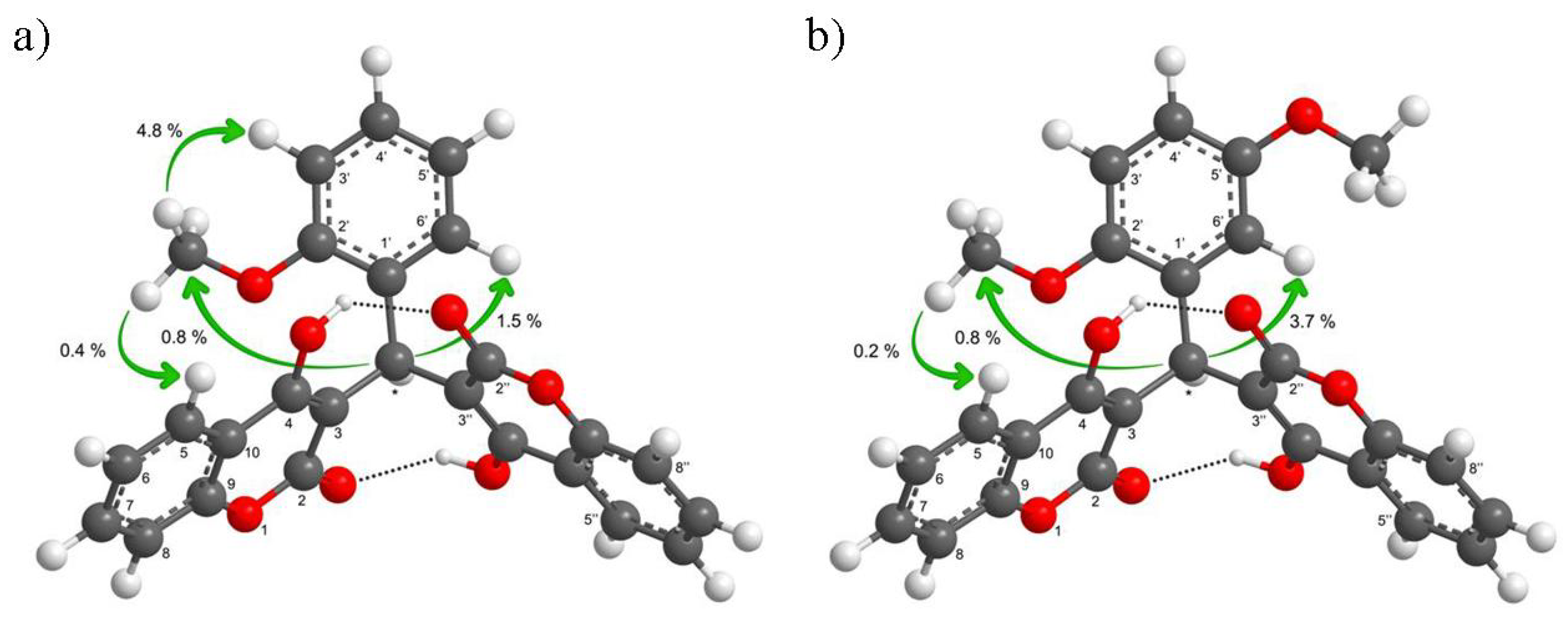
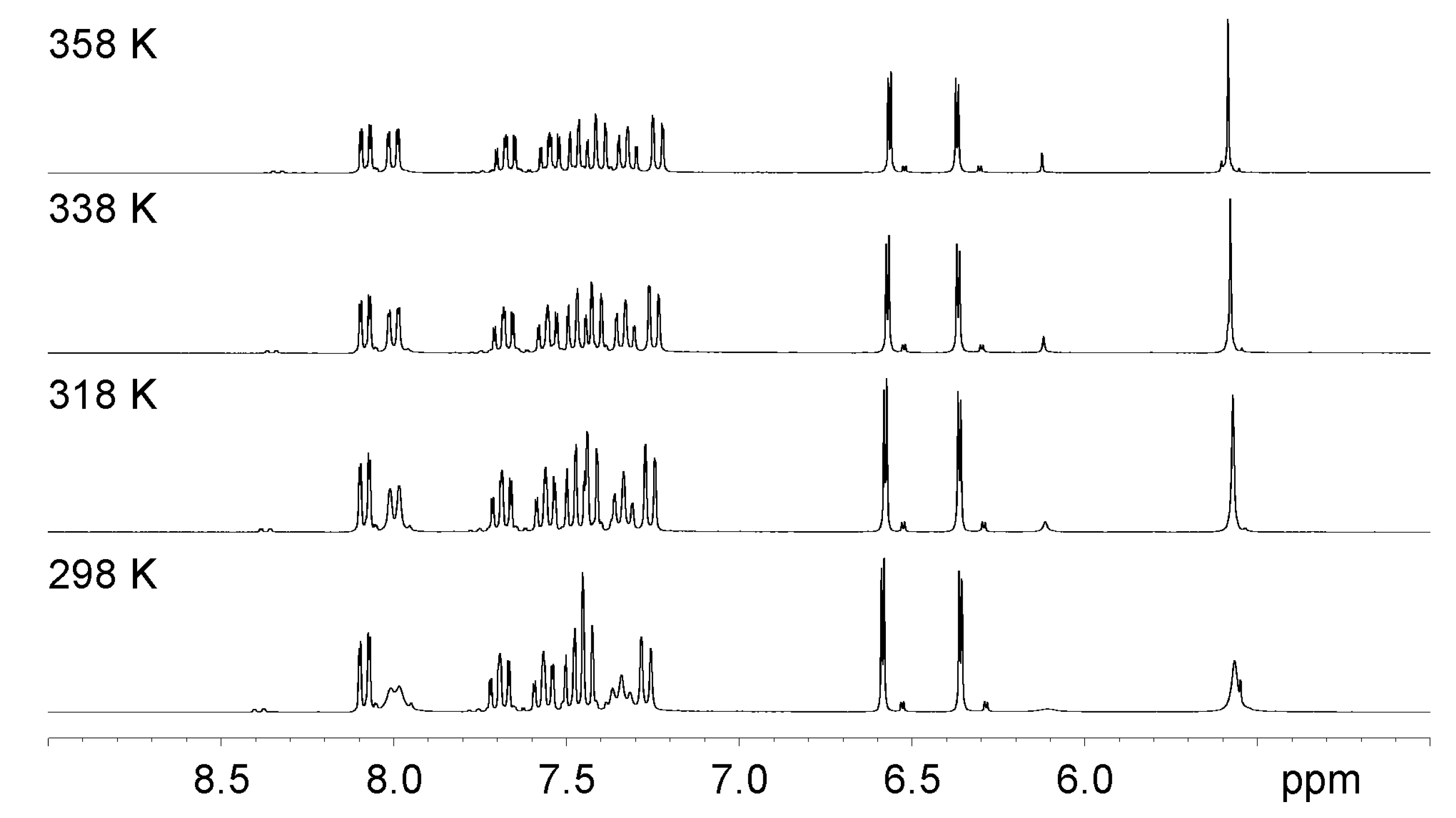
2.2. NMR Assignment and Conformational Study
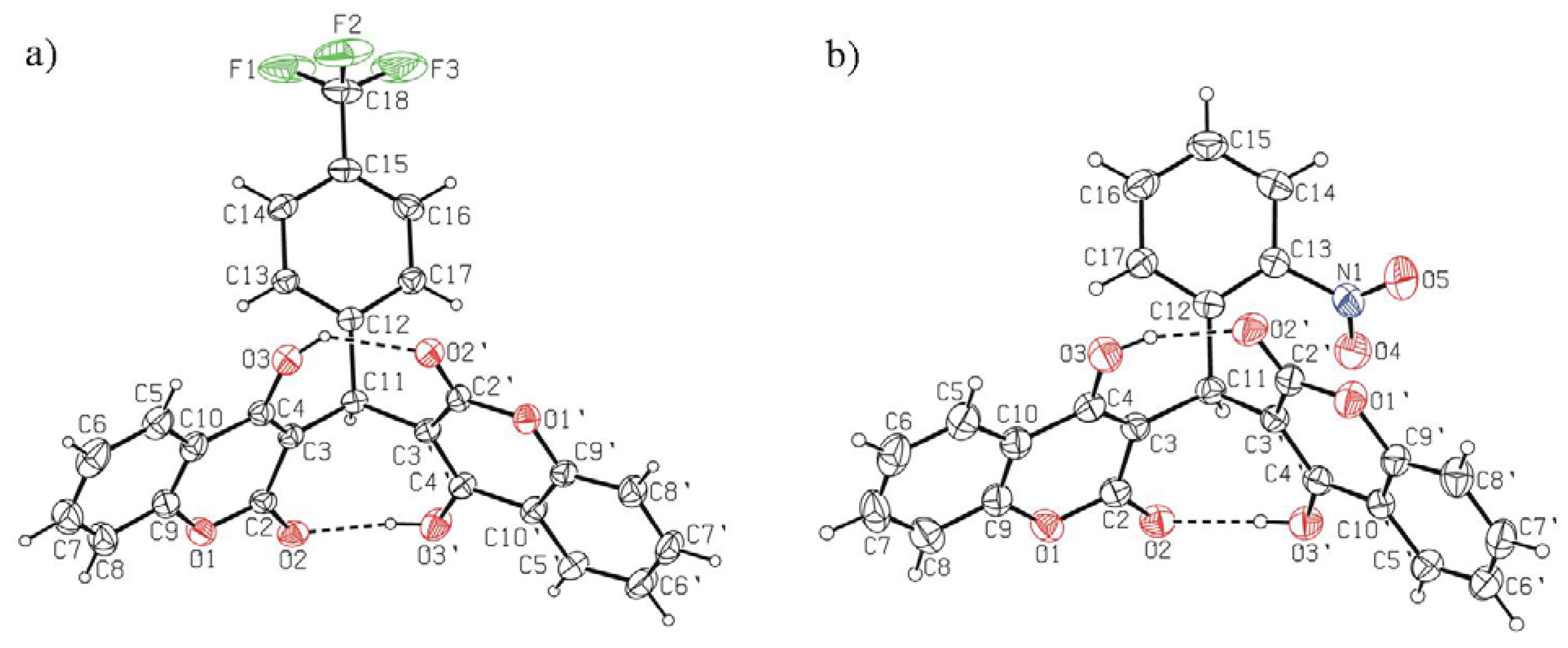
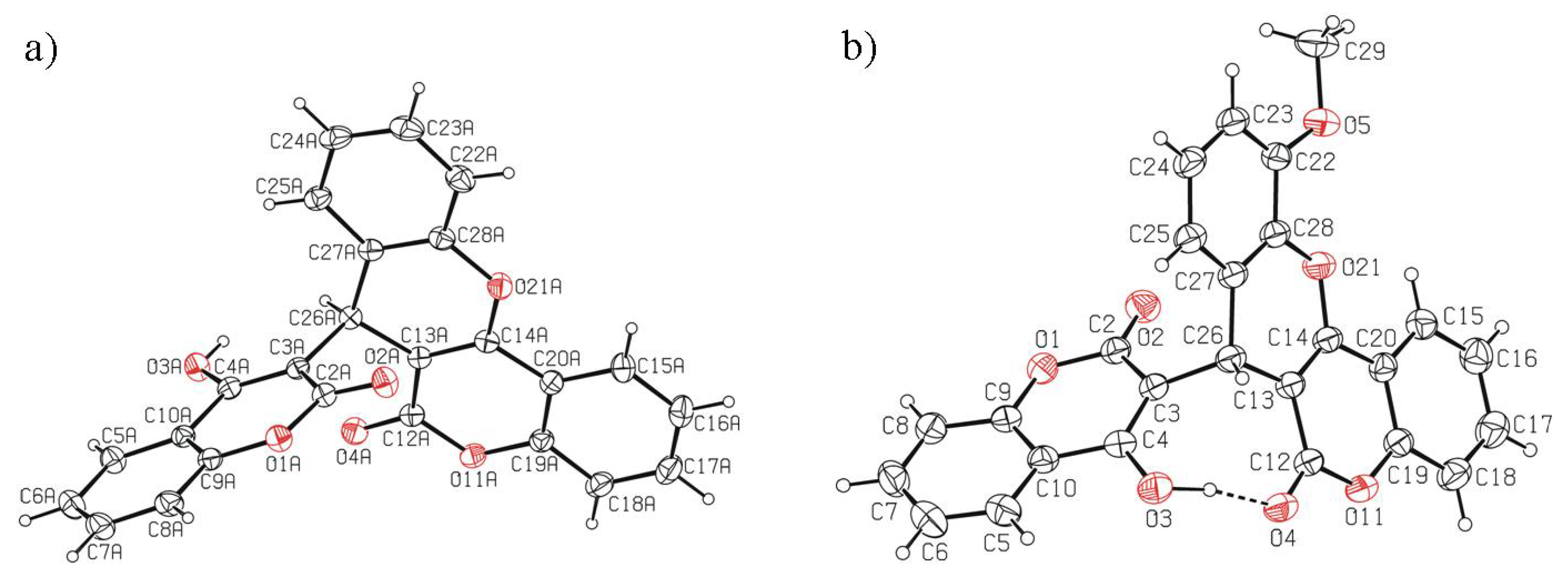
2.3. X-Ray Crystal Structure Study
2.4. Biological Activity Results
3. Experimental
3.1. General Methods
3.2. Procedures for the Preparation of Compounds
3.2.1. The compounds 1–20 were prepared by the following general procedure
3.2.2. Compound data
4.3. Crystal Structure Determination of 7, 9, 16 and 18
4.4. Virological Assays
4. Conclusions
Electronic Supplementary Information (ESI)
Acknowledgments
References and Notes
- Weinmann, I. History of the development and applications of coumarin and coumarin-related compounds. In Coumarins: Biology, Applications and Mode of Action; O’Kennedy, R., Thornes, R.D., Eds.; John Wiley & Sons, Inc.: Los Angeles, CA, USA, 1997; pp. 1–22. [Google Scholar]
- Marcu, M.G.; Schulte, W.T.; Neckers, L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J. Natl. Cancer Inst. 2000, 92, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.C.; Hsu, L.Y.; Tsai, H.J.; Yao, C.W.; Chang, T.C. Synthesis and antimicrobial evaluation of coumarin derivatives. J. Chiong Chang Inst. Techol. 2008, 37, 15–22. [Google Scholar]
- Ćavar, S.; Kovač, F.; Maksimović, M. Synthesis and antioxidant activity of selected 4-methylcoumarins. Food Chem. 2009, 117, 135–142. [Google Scholar] [CrossRef]
- Jung, J.C.; Park, O.S. Synthetic approaches and biological activities of 4-hydroxycoumarin derivatives. Molecules 2009, 14, 4790–4803. [Google Scholar] [CrossRef]
- Symeonidis, T.; Chamilos, M.; Hadjipavlou-Litina, D.J.; Kallitsakis, M.; Litinas, K.E. Synthesis of hydroxycoumarins and hydroxybenzo[f]- or [h]coumarins as lipid peroxidation inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Studying plant-derived coumarins for their pharmacological and therapeutic properties as potential anticancer drugs. Expert Opin. Drug Disc. 2007, 2, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Nolan, A.K.; Doncaster, R.J.; Dunstan, S.M.; Scot, A.K.; Frenkel, D.; Siegel, D.; Ross, D.; Barnes, J.; Levy, C.; Leys, D.; et al. Synthesis and biological evaluation of coumarin-based inhibitors of NAD(P)H: quinone oxidoreductase-1 (NQO1). J. Med. Chem. 2009, 57, 7142–7156. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, D.H.; Pannecouque, C.; De Clercq, E.; Chikhalia, K.H. Synthesis and studies of new 2-(coumarin-4-yloxy)-4,6-(substituted)-s-triazine derivatives as potential anti-HIV agents. Arch. Pharm. 2009, 342, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Neamati, N.; Hong, H.; Mazumder, A.; Wang, S.; Sunder, S.; Milne George, W.A.; Pommier, Y.; Burke, T.R., Jr. Coumarin-based inhibitors of HIV integrase. J. Med. Chem. 1997, 40, 242–249. [Google Scholar] [CrossRef] [PubMed]
- François, K.O.; Auwerx, J.; Schols, D.; Balzarini, J. Simian immunodeficiency virus is susceptible to inhibition by carbohydrate-binding agents in a manner similar to that of HIV: Implications for further preclinical drug development. Mol. Pharmacol. 2008, 74, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Manolov, I.; Danchev, N.D. Synthesis and pharmacological investigations of some 4-hydroxycoumarin derivatives. Arch. Pharm. 2003, 336, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Manolov, I.; Danchev, N.D. Synthesis, toxicological and pharmacological assessment of some 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 1995, 30, 531–535. [Google Scholar] [CrossRef]
- Mazumder, A.; Wang, S.; Neamati, N.; Niklaus, M.; Sunder, S.; Chen, J.; Milne, G.W.A.; Rice, W.G.; Burke, T.R., Jr.; Pommier, Y. Antiretroviral agents as inhibitors of both human immunodeficiency virus type 1 integrase and protease. J. Med. Chem. 1996, 39, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Momekov, G.; Tzanova, T.; Karaivanova, M. Synthesis, characterization, and cytotoxic activity of new lanthanum(III) complexes of bis-coumarins. Bioorg. Chem. Appl. 2006. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.B.; Krishnaswamy, N.R.; Seshadri, T.R.; Seth, S.D.S.; Sharma, B.R. Structure and anticoagulant activity of bridge-substituted dicoumarols. J. Med. Chem. 1967, 10, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Manolov, I.; Maichle-Mössmer, C.; Nicolova, I.; Danchev, N. Synthesis and anticoagulant activities of substituted 2,4-diketochromans, biscoumarins, and chromanocoumarins. Arch. Pharm. 2006, 339, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Manolov, I.; Maichle-Mössmer, C. Synthesis and structure of 3,3′-[(4-bromophenyl)methylene]bis-[4-hydroxy-2H-1-benzopyran-2-one]. Anal. Sci. 2007, 23, x63–x64. [Google Scholar] [CrossRef]
- Valente, E.J.; Eggleston, D.S. Structure of (phenyl)bis(4-hydroxybenzo-2H-pyran-2-one-3-yl)methane. Acta Crystallogr. 1989, C45, 785–787. [Google Scholar] [CrossRef]
- Stanchev, S.; Maichle-Mössmer, C.; Manolov, I. Synthesis, structure and acid-base behaviour of some 4-hydroxycoumarin derivatives. Z. Naturforsch.(B) 2007, 62, 737–741. [Google Scholar] [CrossRef]
- Oxford Diffraction, Xcalibur CCD System. CrysAlisPro; Oxford Diffraction Ltd: Abingdon, England, UK, 2010. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Compound | MCC a (μM) | EC50 b (μM) | ||||
|---|---|---|---|---|---|---|
| HSV-1 (KOS) | HSV-2 (G) | Vaccinia virus | Vesicular stomatitis virus | HSV-1 TK− KOS ACVr | ||
| 3 | >20 | 9 | 9 | 11 | >20 | 9 |
| 7 | 20 | >4 | >4 | >4 | >4 | >4 |
| 8 | 100 | >20 | >20 | >20 | >20 | >20 |
| 11 | 100 | >20 | >20 | >20 | >20 | >20 |
| 13 | 100 | >20 | >20 | >20 | >20 | >20 |
| 14 | >100 | >100 | >100 | >100 | >100 | >100 |
| 15 | >100 | >100 | >100 | >100 | >100 | >100 |
| 19 | >100 | 50 | 45 | >100 | >100 | 50 |
| 20 | 100 | >20 | >20 | >20 | >20 | >20 |
| Brivudin | >250 | 0.06 | 182 | 3.2 | >250 | 50 |
| Cidofovir | >250 | 0.9 | 1.5 | 7.9 | >250 | 1.5 |
| Acyclovir | >250 | 0.30 | 0.35 | >250 | >250 | 20 |
| Ganciclovir | >100 | 0.025 | 0.030 | >100 | >100 | 0.1 |
| Compound | Minimum cytotoxic conc. in CRFK cell cultures (CC50) a (μM) | EC50 b (μM) | |
|---|---|---|---|
| Feline Corona Virus (FIPV) | Feline Herpes Virus | ||
| 1 | 43 | 7.5 | 9.1 |
| 4 | 45 | 9.1 | 7.6 |
| 5 | 36 | 7.5 | 5.0 |
| 6 | 39 | 7.7 | 8.1 |
| 8 | 41 | 8.5 | 6.9 |
| 9 | 34 | 8.3 | 14 |
| 20 | 27 | 6.4 | 7.1 |
| HHA | > 2 | 0.34 | 0.15 |
| UDA | >10 | 4.2 | 7.1 |
| Ganciclovir | >100 | >100 | 4.9 |
| Compound | 7 | 9 | 16 | 18 |
|---|---|---|---|---|
| Formula | C26H15F3O6 | C25H15NO8 | C54H42O15 | C26H16O7 |
| Formula weight | 480.38 | 457.38 | 930.88 | 440.39 |
| Crystal size [mm] | 0.44 × 0.24 × 0.22 | 0.28 × 0.24 × 0.20 | 0.62 × 0.35 × 0.29 | 0.48 × 0.14 × 0.09 |
| Crystal colour, shape | colourless, block | colourless, block | colourless, prism | colourless, prism |
| Crystal system | monoclinic | monoclinic | monoclinic | triclinic |
| Space group | P 21/c | C 2/c | P 21/c | P |
| Unit cell dimensions | ||||
| a [Å] | 10.4430(4) | 34.4429(19) | 18.6160(10) | 9.4927(4) |
| b [Å] | 10.4031(4) | 7.5448(3) | 15.2918(5) | 9.7660(4) |
| c [Å] | 20.1801(9) | 16.8941(9) | 17.7112(10) | 11.8741(5) |
| α [°] | 90 | 90 | 90 | 66.322(4) |
| β [°] | 91.423(3) | 112.749(6) | 115.484(7) | 73.399(4) |
| γ [°] | 90 | 90 | 90 | 83.084(3) |
| V [Å3] | 2191.68(15) | 4048.7(3) | 4551.3(4) | 966.09(7) |
| Z | 4 | 8 | 4 | 2 |
| Dcalc. [g cm−3] | 1.456 | 1.501 | 1.359 | 1.514 |
| μ [mm−1] | 0.120 | 0.114 | 0.100 | 0.111 |
| θ range [°] | 4.14 to 26.00 | 4.19 to 26.00 | 4.16 to 26.00 | 4.21 to 26.00 |
| Collected reflections No. | 17378 | 21957 | 41605 | 13789 |
| Independent reflections No. / Rint. | 4298 / 0.0279 | 3970 / 0.0229 | 8906 / 0.0477 | 3777 / 0.0315 |
| Reflections number I ≥ 2σ(I) | 2649 | 2694 | 5836 | 2341 |
| Data / restraints / parameters | 4298 / 20 / 324 | 3970 / 2 / 315 | 8906 / 6 / 666 | 3777 / 1 / 303 |
| Goodness-of-fit on F2 | 0.999 | 1.010 | 1.020 | 0.997 |
| R [I ≥ 2σ(I)] / R [all data] | 0.0611 / 0.0926 | 0.0391 / 0.0587 | 0.0453 / 0.0878 | 0.0390 / 0.0707 |
| wR [I ≥ 2σ(I)] / wR [all data] | 0.2004 / 0.2150 | 0.1103 / 0.1158 | 0.1039 / 0.1305 | 0.0954 / 0.1043 |
| Max./min. elect. density [e Å−3] | 0.543 / −0.425 | 0.388/ −0.186 | 0.189 / −0.270 | 0.216 / −0.174 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Završnik, D.; Muratović, S.; Makuc, D.; Plavec, J.; Cetina, M.; Nagl, A.; Clercq, E.D.; Balzarini, J.; Mintas, M. Benzylidene-bis-(4-Hydroxycoumarin) and Benzopyrano-Coumarin Derivatives: Synthesis, 1H/13C-NMR Conformational and X-ray Crystal Structure Studies and In Vitro Antiviral Activity Evaluations. Molecules 2011, 16, 6023-6040. https://doi.org/10.3390/molecules16076023
Završnik D, Muratović S, Makuc D, Plavec J, Cetina M, Nagl A, Clercq ED, Balzarini J, Mintas M. Benzylidene-bis-(4-Hydroxycoumarin) and Benzopyrano-Coumarin Derivatives: Synthesis, 1H/13C-NMR Conformational and X-ray Crystal Structure Studies and In Vitro Antiviral Activity Evaluations. Molecules. 2011; 16(7):6023-6040. https://doi.org/10.3390/molecules16076023
Chicago/Turabian StyleZavršnik, Davorka, Samija Muratović, Damjan Makuc, Janez Plavec, Mario Cetina, Ante Nagl, Erik De Clercq, Jan Balzarini, and Mladen Mintas. 2011. "Benzylidene-bis-(4-Hydroxycoumarin) and Benzopyrano-Coumarin Derivatives: Synthesis, 1H/13C-NMR Conformational and X-ray Crystal Structure Studies and In Vitro Antiviral Activity Evaluations" Molecules 16, no. 7: 6023-6040. https://doi.org/10.3390/molecules16076023
APA StyleZavršnik, D., Muratović, S., Makuc, D., Plavec, J., Cetina, M., Nagl, A., Clercq, E. D., Balzarini, J., & Mintas, M. (2011). Benzylidene-bis-(4-Hydroxycoumarin) and Benzopyrano-Coumarin Derivatives: Synthesis, 1H/13C-NMR Conformational and X-ray Crystal Structure Studies and In Vitro Antiviral Activity Evaluations. Molecules, 16(7), 6023-6040. https://doi.org/10.3390/molecules16076023




