A General and Simple Diastereoselective Reduction by L-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides
Abstract
:1. Introduction


2. Results and Discussion

| Entry | R | Yield [%] a | syn/anti ratio |
|---|---|---|---|
| 1 | CH3 (10a) | 93 | 6:1 b |
| 2 | C2H5 (10b) | 97 | 7:1 c |
| 3 | n-C4H9 (10c) | 97 | 7:1 c |
| 4 | n-C5H11 (10d) | 95 | 23:2 c |
| 5 | n-C8H17 (10e) | 98 | 23:2 b |
| 6 | n-C12H25 (10f) | 85 | 9:1 b |
| 7 | n-C16H33 (10g) | 83 | 7:1 b |
| 8 | i-Bu (10h) | 92 | 3:1 c |
| 9 | Ph (10i) | 81 | 3:1 b |
| 10 | Bn (10j) | 92 | 11:2 c |
| 11 | PhCH2CH2 (10k) | 82 | 7:2 c |

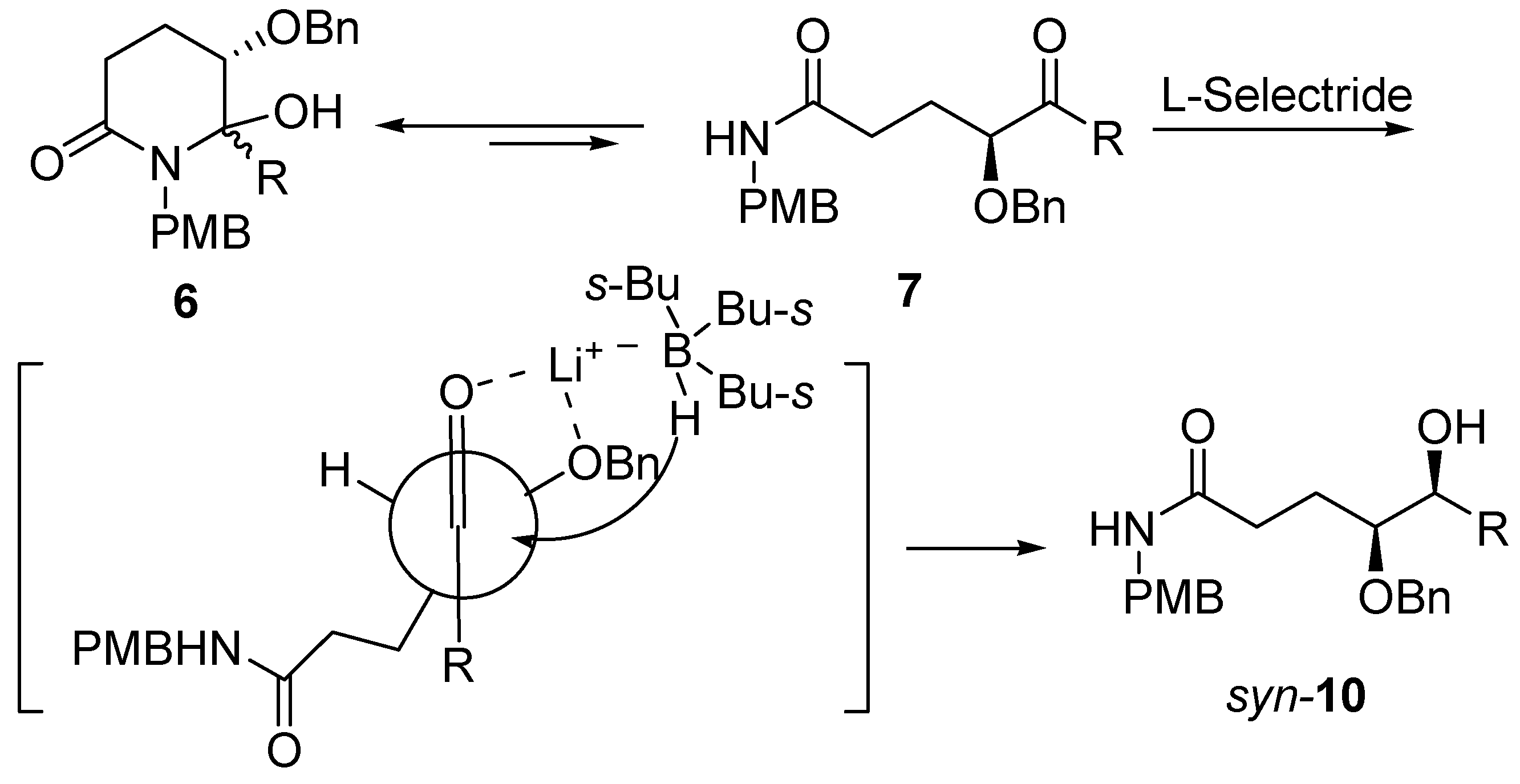
3. Conclusions
4. Experimental
4.1. General methods
4.2. General procedure for preparation of syn-10
4.3. The synthesis of (5S,6R)-2-Piperidinone 8a via the cyclization of 10a
Acknowledgements
References and Notes
- Ratnayake, A.S.; Yoshida, W.Y.; Moonberry, S.L.; Hemscheidt, T. The structure of Microcar-palide, a Microfilament disrupting agent from an endophytic fungus. Org. Lett. 2001, 3, 3479–3481. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Souza, M.L.M.; Ibrahim, T.; Melo, G.O.; Almeida, A.P.; Guette, C.; Férézou, J.P.; Koatz, V.L.G. Kalanchosine dimalate, an anti-inflammatory salt from Kalanchoe brasiliensis. J. Nat. Prod. 2006, 69, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Mcinerney, B.V. Biologically active metabolites from xenorhabdus spp. Part 2. Benzopyran-1-one derivatives with gastroprotective activity. J. Nat. Prod. 1991, 54, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, M.; Kuwahara, S. Concise synthesis of PM-94128 and Y-05460M-A. J. Org. Chem. 2009, 74, 7566–7599. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, Y.; Hayashi, H.; Ooka, T.; Shibukawa, M.; Iitaka, Y. Studies on AI-77s, microbial products with gastroprotective activity. Structures and the chemical nature of AI-77s. Tetrahedron 1984, 40, 2519–2527. [Google Scholar] [CrossRef]
- Shimojima, Y.; Shirai, T.; Baba, T.; Hayashi, H. 1H-2-Benzopyran-1-one derivatives, microbial products with pharmacological activity. Conversion into orally active derivatives with antiinflammatory and antiulcer activities. J. Med. Chem. 1985, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, Y.; Hayashi, H. 1H-2-Benzopyran-1-one derivatives, microbial products with pharmacological activity. Relationship between structure and activity in [[1(S)-(3(S), 4-dihydro-8-hydroxy-l-oxo-1H-2-benzopyran-3-yl)-3-methylbutylamino]-4(S),5(S)-dihydroxy-6-oxo-3(S)-ammoniohexanoate. J. Med. Chem. 1983, 26, 1370–1374. [Google Scholar] [PubMed]
- Murga, J.; Falomir, E.; Garcoa-Fortanet, J.; Carda, M.; Marco, J.A. Stereoselective synthesis of Microcarpalide. Org. Lett. 2002, 4, 3447–3449. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, M.K.; Nagaprasad, R.; Ramana, C.V. Total synthesis of microcarpalide. Tetrahedron Lett. 2003, 44, 2873–2875. [Google Scholar] [CrossRef]
- Banwell, M.G.; Loong, D.T.J. Stereoselective total synthesis of the nonenolide (+)-microcarpalide. Heterocycles 2004, 62, 713–734. [Google Scholar] [CrossRef]
- Gurjar, M.K.; Nagaprasad, R.; Ramana, C.V.; Karmakar, S.; Moha patra, D.K. Ring-closing metathesis mediated total synthesis of microcarpalide and herbarumin III. ARKIVOC 2005, (iii), 237–257. [Google Scholar]
- García-Fortanet, J.; Murga, J.; Falomir, E.; Carda, M.; Marco, J.A. Stereoselective total synthesis and absolute configuration of the natural decanolides (-)-Microcarpalide and (+)-Lethaloxin. Identity of (+)-Lethaloxin and (+)-Pinolidoxin. J. Org. Chem. 2005, 70, 9822–9827. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rao, R.V.; Shashidhar, J. Total synthesis of (-)-microcarpalide from D-mannitol. Tetrahedron Lett. 2005, 46, 5479–5481. [Google Scholar] [CrossRef]
- Ishigami, K.; Kitahara, T. Synthesis of microcarpalide, a microfilament disrupting agent. Heterocycles 2004, 63, 785–790. [Google Scholar] [CrossRef]
- Ishigami, K.; Watanabe, H.; Kitahara, T. Stereoselective synthesis of microcarpalide. Tetrahedron 2005, 61, 7546–7553. [Google Scholar] [CrossRef]
- Davoli, P.; Fava, R.; Morandi, S.; Spaggiari, A.; Prati, F. Enantioselective total synthesis of (-)-microcarpalide. Tetrahedron 2005, 61, 4427–4436. [Google Scholar] [CrossRef]
- Chavan, S.P.; Praveen, C. Stereoselective synthesis of (-)-microcarpalide. Tetrahedron Lett. 2005, 46, 1939–1941. [Google Scholar] [CrossRef]
- Kumar, P.; Naidu, S.V. Total synthesis of microcarpalide. J. Org. Chem. 2005, 70, 4207–4210. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-Q.; Liu, L.-X.; Wei, B.-G.; Ruan, Y.-P. Asymmetric synthesis of (+)-l-733,060 and (+)-CP-99,994 based on a new chiral 3-piperidinol synthon. Org. Lett. 2003, 5, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-X.; Ruan, Y.-P.; Guo, Z.-Q.; Huang, P.-Q. A general approach to (5S,6R)-6-alkyl-5-benzyloxy-2-piperidinones: application to the asymmetric syntheses of neurokinin substance P peceptor antagonist (-)-l-733,061 and (-)-deoxocassine. J. Org. Chem. 2004, 69, 6001–6009. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-X.; Huang, P.-Q. SN2 reaction of 2-substituted 3-piperidinol mesylate with retention of conguration: application to the asymmetric synthesis of (2R,3S)-CP-99,994. Tetrahedron: Asymmetry 2006, 17, 3265–3272. [Google Scholar] [CrossRef]
- Liu, L.-X.; Pen, Q.-L.; Huang, P.-Q. A new approach for the asymmetric syntheses of (2S,3S)-3-hydroxypipercolic acid. Tetrahedron Asymmetry 2008, 19, 1200–1203. [Google Scholar] [CrossRef]
- Yu, D.-S.; Xu, W.-X.; Liu, L.-X.; Huang, P.-Q. First asymmetric synthesis of piperidine alkaloid (‑)-morusimic acid. Synlett 2008, 1189–1192. [Google Scholar]
- Liu, L.-X.; Xiao, K.J.; Huang, P.-Q. Chemo- and diastereoselective control for a flexible approach to (5S,6S)-6-alkyl-5-benzyloxy-2-piperidinones. Tetrahedron 2009, 65, 3834–3841. [Google Scholar] [CrossRef]
- Xiao, K.J.; Liu, L.-X.; Huang, P.-Q. An enantioselective of synthesis of (+)-azimic acid. Tetrahedron Asymmetry 2009, 20, 1181–1184. [Google Scholar] [CrossRef]
- García Ruano, J.L.; Fernández-Ibáñez, M.Ά.; Maestro, M.C.; Rodríguez-Fernández, M.M. Remote stereocontrol by sulfinyl groups: reduction of δ-ketosulfoxides. J. Org. Chem. 2005, 70, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Bahia, P.S.; Snaith, J.S. Synthesis of 3-substituted 4-piperidinones via a one-pot Tandem oxidation-cyclization-oxidation process: stereodivergent reduction to 3,4-disubstituted piperidines. J. Org. Chem. 2004, 69, 3226–3229. [Google Scholar] [CrossRef] [PubMed]
- Colyer, J.T.; Andersen, N.G.; Tedrow, J.S.; Soukup, T.S.; Faul, M.M. Reversal of diastereofacial selectivity in hydride reductions of N-tert-butanesulfinyl imines. J. Org. Chem. 2006, 71, 6859–6862. [Google Scholar] [CrossRef] [PubMed]
- Ripoche, I.; Bennis, K.; Canet, J.-L.; Gelas, J.; Troin, Y. Diastereoselective elaboration of 2,3,4-substituted piperidines using diene iron tricarbonyl complexes. Total synthesis of (±)-dienomycin C and (±)-4-epi-dienomycin C. Tetrahedron Lett. 1996, 37, 3991–3992. [Google Scholar] [CrossRef]
- Mohanta, P.K.; Davis, T.A.; Gooch, J.R.; Flowers, R.A., II. Chelation-controlled diastereoselective reduction of α-fluoroketones. J. Am. Chem. Soc. 2005, 127, 11896–11897. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.M.; Barrio, R.; Martínez, M.A.; Pedrosa, R.; Pérez-Encabo, A. Synthesis of enantiopure syn-β-amino alcohols. A simple case of chelation-controlled additions of diethylzinc to α-(dibenzylamino) aldehydes. J. Org. Chem. 1996, 61, 4210–4213. [Google Scholar]
- Marco, J.A.; Carda, M.; González, F.; Rodríguez, S.; Castillo, E.; Murga, J. J. Org. Chem. 1998, 63, 698–707. [CrossRef] [PubMed]
- Cram, D.J.; Abd Elhafez, F.A. Studies in stereochemistry. X. The rule of “steric control of asymmetric induction” in the syntheses of acyclic systems. J. Am. Chem. Soc. 1952, 74, 5828–5835. [Google Scholar] [CrossRef]
- Cram, D.J.; Kopecky, K.R. Studies in stereochemistry. XXX. models for steric control of asymmetric. J. Am. Chem. Soc. 1959, 81, 2748–2755. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 10a-10k are available from the authors. |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yin, B.; Ye, D.-N.; Yu, K.-H.; Liu, L.-X. A General and Simple Diastereoselective Reduction by L-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides. Molecules 2010, 15, 2771-2781. https://doi.org/10.3390/molecules15042771
Yin B, Ye D-N, Yu K-H, Liu L-X. A General and Simple Diastereoselective Reduction by L-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides. Molecules. 2010; 15(4):2771-2781. https://doi.org/10.3390/molecules15042771
Chicago/Turabian StyleYin, Bo, Dong-Nai Ye, Kai-Hui Yu, and Liang-Xian Liu. 2010. "A General and Simple Diastereoselective Reduction by L-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides" Molecules 15, no. 4: 2771-2781. https://doi.org/10.3390/molecules15042771




