Abstract
Some novel β-biarylacryl ferrocene derivatives were synthesized via Pd-catalytic Suzuki cross-coupling reactions of β-(2-bromophenyl)acrylferrocene and arylboronic acids. The structures were determined with elemental analyses, IR spectra, and 1H-NMR spectra.
Introduction
Over the last two decades, molecular-based second-order nonlinear optical (NLO) chromophores have attracted much interest because of their potential applications in emerging opto-electronic technologies [1,2,3,4,5]. These efforts have mainly focused on organic systems. More recently, organometallic molecules have been investigated as well [3,4,5]. In comparison to common organic molecules, they offer a large variety of novel structures. The possibility of high environmental stability and diversity of tunable electronic behaviors by virtue of the coordinated metal center might bring about NLO materials with unique characteristics such as magnetic and electro-chemical properties.
In the present communication, we continue our previous research work [6] and wish to report the synthesis by Suzuki cross-coupling reaction [7] of some novel β-biarylacryl ferrocene organometallic structures having potential electro-active material properties.
Results and Discussion
Synthesis of the biaryl organometallic structures having an extended conjugation system was satisfactorily carried out starting from acetylferrocene, prepared from ferrocene using a literature method [8]. β-(2-Bromophenyl)acrylferrocene (3) was prepared by a base catalyzed Claisen condensation reaction of acetylferrocene with 2-bromobenzaldehyde at ambient temperature, the product obtained in practically quantitative yield [9] was mainly in the E-isomer form (the Z-isomer being separated off by recrystallization in ethyl alcohol). The catalytic cross-coupling reaction of β-(2-bromophenyl)acrylferrocene (3) with arylboronic acids to prepare the biaryl analogues permits the extension of molecular conjugation. Thus, compound 3 was transformed into β-biarylacrylferrocenes 4 by Suzuki cross-coupling reaction with arylboronic acids in the presence of Pd(0) in a sodium carbonate solution. The new compounds were separated in good yield by column chromatography with 4:1 to 30:1 petroleum ether-diethyl ether as eluants. The reactions are shown in Scheme 1.
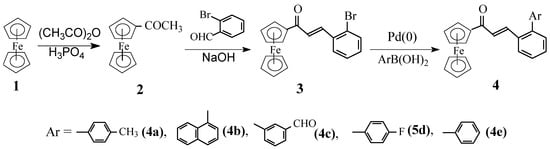
Scheme 1.
Scheme 1.
Conclusions
A novel organometallic compound structure, β-biarylacryl ferrocenes, are synthesized by [tetrakis(triphenylphosphine)palladium] catalyzed Suzuki cross-coupling reactions of β-(2-bromo- phenyl)acrylferrocene and arylboronic acids. Further studies will be needed to better understand the scope that the different factors affecting this reaction, and how the coupling reaction yields can be improved.
Experimental
General
Melting points were determined with a Thomas Hoover capillary melting point apparatus and are uncorrected. IR spectra were recorded on a FT-170 SX spectrometer as KBr pellets. 1H-NMR spectra were measured on a Bruker AM-400 FT-NMR spectrometer in CDCl3. Chemical shifts are referred to TMS on the δ scale. Elemental analyses were performed on a Carlo Erba 1106 instrument. Phenylboronic acid [10], naphylthylboronic acid [10], 4-methylphenylboronic acid [10], 3-formylphenylboronic acid [11], 4-fluorophenylboronic acid [12], and the Pd-catalyst [13] were prepared by literature procedures.
β-(2-Bromophenyl)acrylferrocene (3).
Acetylferrocene (2, 5.7 g, 0.025 mole) was dissolved in 95% ethyl alcohol (20 mL) and a solution of o-bromobenezaldehyde (4.83 g, 0.026 mole) in 95% ethyl alcohol (6 mL) was added to the above mixture with stirring. After that, stirring was continued for half an hour until the solid was dissolved. A solution of 95% ethyl alcohol (20 mL), water (20 mL), and sodium hydroxide (2.0 g, 0.05 mole) was added dropwise to above mixture under stirring at room temperature. A reddish precipitate appeared after a few minutes, and became difficult to stir. After two hours TLC indicated the reaction was complete (Rf = 0.66, ethyl acetate/petroleum ether 1:4 v:v). The mixture was poured into water (200 ml) and neutralized to pH=7 with 2M HCl, cooled and filtered, the solid washed with water (3×20 mL) and dried under vacuum. The crude product was recrystallized from chloroform to afford 7.6 g (90%) of 3 as dark claret colored crystals. Anal. For C19H15BrFeO: Found (%): C 57.58, H 3.92, Calcd. (%) C57.76, H 4.05; M.p.: 122-124oC; 1H-NMR: 7.82 (d, 1H, J=15.70 Hz, H-3), 7.78 (d, 1H, J=8.35 Hz, H-3′), 7.46-7.37 (m, 3H, H-4′,5′,6′), 6.98 (d, 1H, J=15.65 Hz, H-2), 4.83 (s, 2H, H-2′″,5′″), 4.56 (s, 2H, H-3′″,4′″), 4.18 (s, 5H, H-6′″); IR (cm-1): 3087, 1650, 1587, 1455, 1376, 1080, 1000, 767, 502, 478.
General procedure for preparation of the title compounds 4a-e.
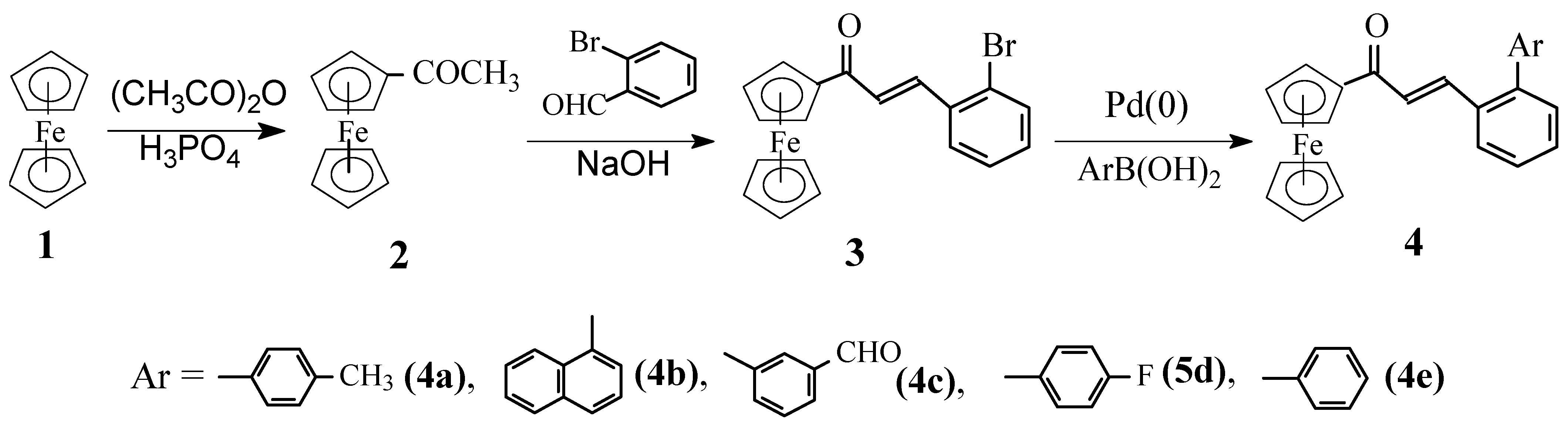
A 50 mL three-necked round-bottomed flask was charged a under N2 atmosphere with β-(2-bromophenyl)acrylferrocene (3, 4.2 mmol, 1.66 g), Pd(0) [tetrakis (triphenylphosphine)palladium] (139 mg, 0.12 mmol, 3 mole% of the substrate), benzene (20 mL), and 2M aqueous sodium carbonate solution (5 mL). The mixture was stirred vigorously, then the appropriate arylboronic acid (4.2 mmol) was added. The reaction mixture was heated to reflux with stirring under N2 atmosphere for an appropriate time, monitored with TLC. After completion, the mixture was cooled to room temperature and was diluted with dichloromethane (60 mL) and water (40 mL). The organic layer was separated, washed with water and brine, dried, and the solvents were removed under reduced pressure. The crude product was purified by column chromatography with petroleum ether-diethyl ether=4:1 to 30:1 (v:v) as eluants. The first red band of starting materials was retrieved, then the second red-band was collected, and the solvents were removed under vacuum to afford pure products 4 with the structures shown in Scheme 2.
Scheme 2.
Scheme 2.
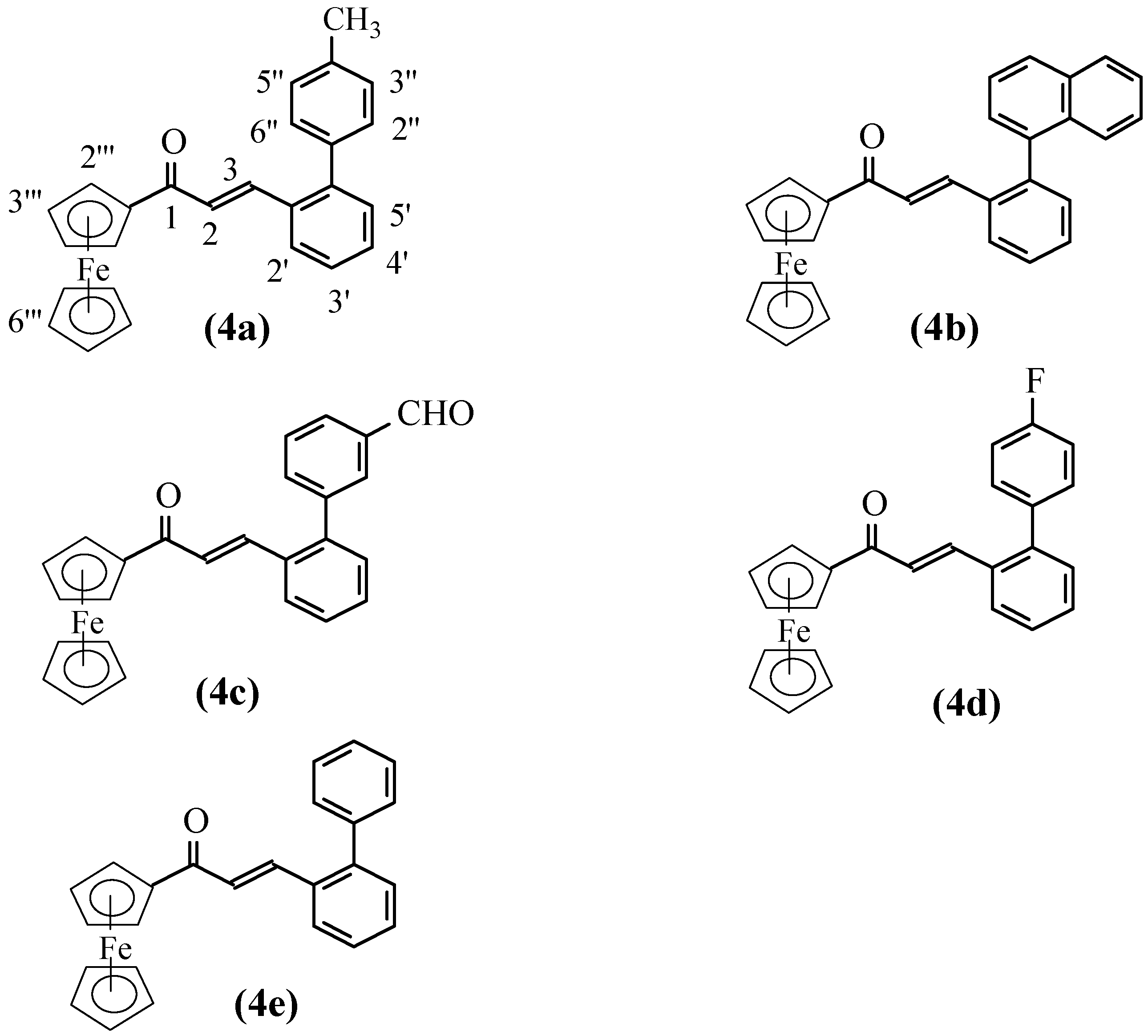
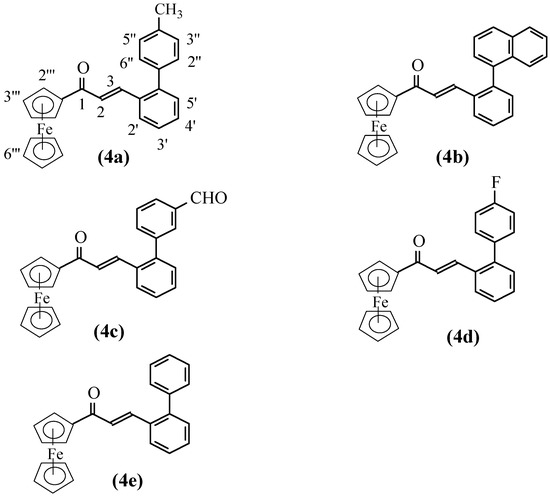
β-[2′-(4″-Methylphenyl)phenyl]acrylferrocene (4a). Yield: 82.9%; Deep red color; M.p.: 138-140 oC; Rf= 0.36 (ethyl acetate-petroleum ether=1:20);Anal. For C26H22FeO: Found (%) C 76.66, H 5.28, Calcd (%): C 76.85, H 5.46; 1H-NMR: 8.08 (d, 1H, J=15.88 Hz, H-3), 7.79 (d, 2H, J=7.44 Hz, H-2″,6″), 7.75(d, 2H, J=7.28 Hz, H-3″,5″), 7.80,7.45-7.38 (m, 4H, H-3′,4′,5′,6′), 7.02(d, 1H, J= 15.65 Hz, H-2), 4.90(s, 2H, H-2′″,5′″), 4.61(s, 2H, H-3′″,4′″), 4.24(s, 5H, H-6′″), 2.41(s, 3H, CH3); IR (cm-1): 3087, 1650, 1587, 1455, 1376, 1080, 1000, 821, 767, 502, 478.
β-[2′-(Naphthalen-1″-yl)phenyl]acrylferrocene (4b). Yield: 69.2%; Red color; M.p.: 130-132 oC ; Rf=0.29 (ethyl acetate/petroleum ether=1:4); Anal. For C29H22FeO: Found (%) C 78.70, H 4.98; Calcd. (%): C 78.74, H 5.01; 1H-NMR: 7.97-7.37 (m, 11H, Ar-H), 7.43 (d, 1H, J=17.10 Hz, H-3), 6.68 (d, 1H, J=15.75 Hz, H-2), 4.49 (br, 2H, J=5.56 Hz, H-2′″,5′″), 4.42 (s, 2H, H-3′″,4′″), 3.96 (s, 5H, H-6′″); IR (cm-1): 3092, 1650, 1586, 1454, 1078, 1001, 827, 802, 779, 768, 503, 477.
β-[2′-(3″-Formylphenyl)phenyl]acrylferrocene (4c). Yield: 67.6%; Deep red color; M.p.: 142-144 oC; Rf = 0.31 (ethyl acetate-petroleum ether=1:4); Anal. For C26H20FeO2: Found (%) C 74.22, H 4.81; Calcd. (%): C 74.29, H 4.80; 1H-NMR: 10.08 (s, 1H, CHO), 7.93 (br, 1H, H-6′), 7.90 (s, 1H, H-2″), 7.85 (br, 1H, H-3′), 7.78 (d, 1H, J=15.60 Hz, H-3), 7.63 (m, 2H, H-4″,6″), 7.49 (m, 2H, H-4′,5′), 7.41 (br, 1H, H-5″), 7.02 (d, 1H, J=15.26 Hz, H-2), 4.84 (s, 2H, H-2′″,5′″), 4.58 (s, 2H, H-3′″,4′″), 4.19 (s, 5H, H-6′″); IR (cm-1): 3087, 1686, 1643, 1582, 1452, 1081, 990, 900, 817, 764, 700, 502, 479.
β-[2′-(4″-Fluorophenyl)phenyl]acrylferrocene (4d). Yield: 65.8%; Deep red color; M.p.: 152-154 oC; Rf = 0.22 (ethyl acetate-petroleum ether=1:10); Anal. For C25H19FFeO: Found (%) C 73.10, H 4.67, Calcd. (%): C 73,19, H 4.67; 1H-NMR: 7.77 (d, 1H, J=17.25 Hz, H-3), 7.79,7.40-7.30 (br, 4H, H-3′,4′,5′,6′), 7.45 (br, 2-H, H3″,5″), 7.15 (br, 2H, H-2″,6″), 7.02 (d, br, 1H, J=13.20 Hz, H-2), 4.85 (s, br, 2H, H-2′″,5′″), 4.58 (s, br, 2H, H-3′″,4′″), 4.20 (s, br, 5H, H-6′″); IR (cm-1): 3081, 1647, 1583, 1455, 1214, 1158, 1080, 1000, 836, 764, 501, 473.
β-(Biphenyl-2′-yl)acrylferrocene (4e). Yield: 63.0%; Deep red color; M.p.: 139-141 oC; Rf =0.48 (ethyl acetate-petroleum ether=1:16); Anal. for C25H20FeO: Found (%) C 76.42, H 5.10, Calcd. (%): C 76.54, H 5.14; 1H-NMR: 7.80 (d,1H, J=16.75 Hz, H-3), 7.79,7.48-7.35 (m, br, 9H, H-3′,4′,5,′6′, H-2″,3″,4″,5″,6′″), 6.97 (d, 1H, J=15.20 Hz, H-2), 4.82 (s, br, 2H, H-2′″,5′″), 4.55 (s, br, 2H, H-3′″,4′″), 4.17 (s, 5H, H-6′″); IR (cm-1): 3089, 1650, 1585, 1456, 1081, 998, 767, 751, 702, 502, 474.
References
- Green, M. L. H.; Marder, S. R.; Thompson, M. E.; Bandy, J. A.; Bloor, D.; Kolinsky, P. V.; Jones, R. J. Synthesis and Structure of cis-1-Ferrocenyl- 2-(4-nitrophenyl) Ethylene, An Organo- transition Metal Compound with A Large Second-order Optical Non-linearity. Nature 1987, 330, 360. [Google Scholar] [CrossRef]
- Kott, K. L.; Higgins, D. A.; McMahon, R. J.; Corn, R. C. Observation of Photo-induced Electron Transfer at A Liquid-liquid Interface by Optical Second Harmonic Generation. J. Am. Chem. Soc. 1993, 115, 542. [Google Scholar]
- Calabrese, J. C.; Cheng, L.-T.; Green, J. C.; Marder, S. R.; Tam, W. Molecular Second-order Optical Nonlinearities of Metallocenes. J. Am. Chem. Soc. 1991, 13, 7227. [Google Scholar] [CrossRef]
- Yuan, Z.; Stringer, G.; Jobe, I. R.; Kreller, D.; Scot, K.; Koch, L.; Taylor, N. J.; Marder, T. B. Synthesis and Characterization of Ferrocenyl and Bis (ferrocenyl) Alkynes and Polynes: Crystal Structure of 1,4-Bis(ferrocenyl) Butadiyne and Third-order Nonlinear Optical Properties of 1,8-Bis(ferrocenyl) Octatetrayne. J. Organomet. Chem. 1993, 452, 115. [Google Scholar] [CrossRef]
- Long, N. J. Organometallic Compounds for Nonlinear Optics–The Search for En-light-enment. Angew. Chem. Int. Ed. Engl. 1995, 34, 21. [Google Scholar]
- Song, Q-B; Li, X-N; Shen, T-H; Yang, S-D; Qiang, G-R; Wu, X-L.; Ma, Y-X. Synthesis of Novel Chalcone Analogues of Ferrocene Biarenes. Synth. Commun. 2003, 33, 3935. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457. [Google Scholar]
- Graham, P. J.; Lindsey, R. V.; Parshall, G.; Peterson, M. L.; Whitman, G. M. Some Acyl Ferrocenes and their Reactions. J. Am. Chem. Soc. 1957, 79, 3416. [Google Scholar] Rosenblum, M.; Woodward, R. B. The Structure of Chemistry of Ferrocene III. Evidence Pertaining to the Ring Rotational Barrier. J. Am. Chem. Soc. 1958, 80, 5443. [Google Scholar]
- Nagy, A. G.; Marton, J.; Sohar, P. Spectroscopic Investigation of Chalcone- analogous Ferrocenes Ortho-substituted in the Aromatic ring. II. Ferrocenyl- COCH=CH-aryl Type Compounds. Acta Chim. Hung. 1991, 128, 961. [Google Scholar]
- Hawkins, R. T.; Lennarr, W. T.; Snyder, H. R. Arylboronic Acids (V) Methyl-substituted Boronic Acids, and Aryl Borons. J. Am. Chem. Soc. 1960, 82, 3053. [Google Scholar]
- Feulner, H.; Linti, G.; Noth, H. Boron Chemistry 206. Preparation and Structural Charaterization of p-Formylphenylboronic Acid. Chem. Ber. 1990, 123, 1841. [Google Scholar]
- Rodriguez, J.G.; Pleite, S. Synthesis of Controlled π-Extended Conjugate Nanostructures of 1,1’-Ferrocene. J. Organomet. Chem. 2001, 637-639, 230. [Google Scholar]
- Coulson, D. R. Tetrakis(triphenylphosphine)palladlum. Inorg. Synth. 1972, 13, 121. [Google Scholar]
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
