Developments of Chiral Metallocenes as Polymerization Catalysts
Abstract
:Introduction
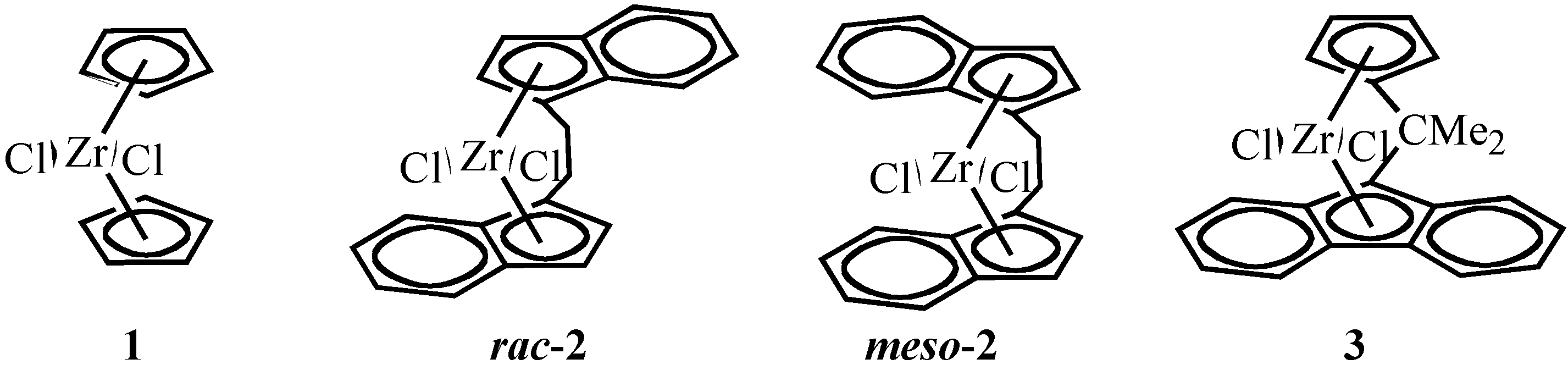
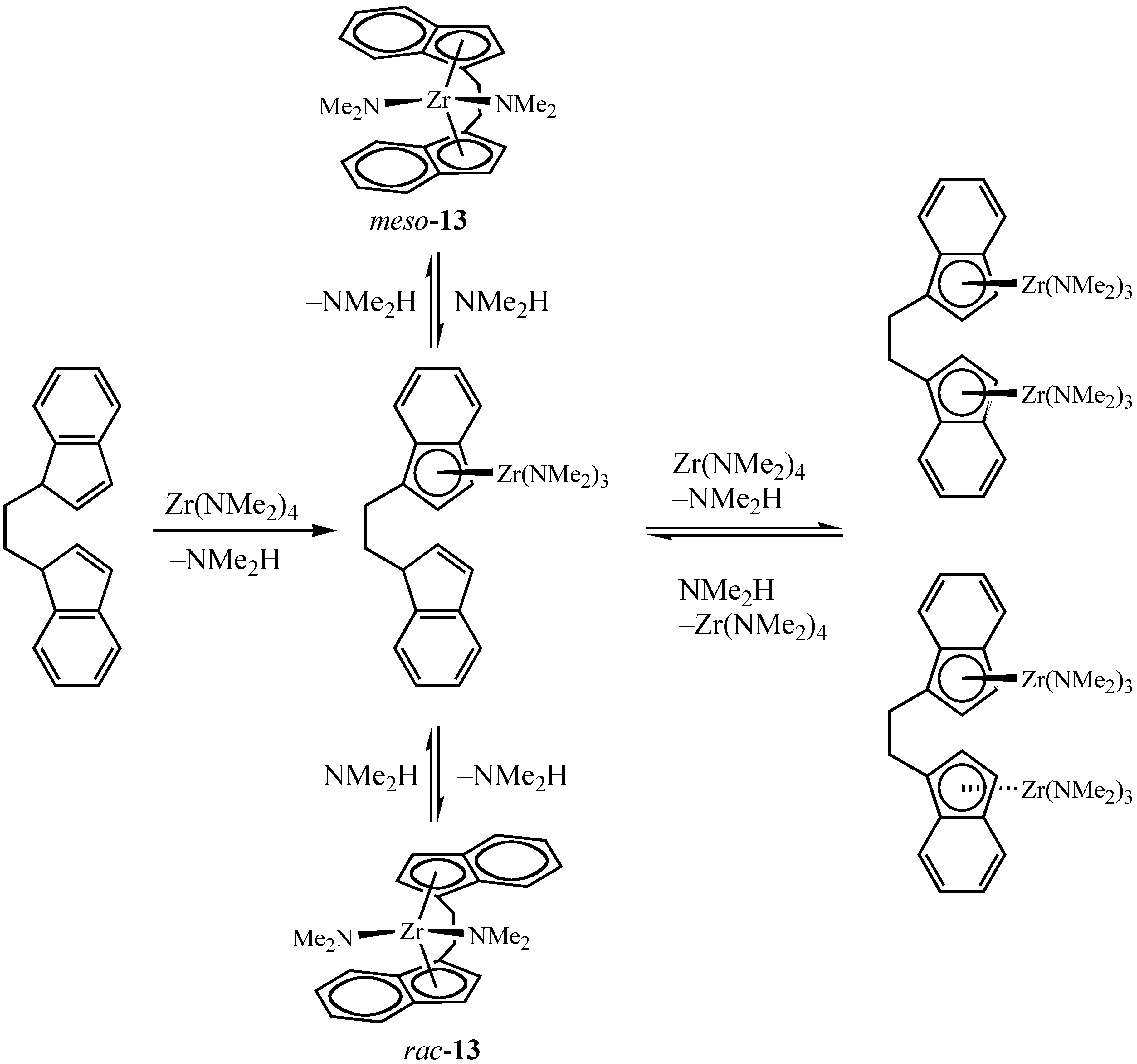
Selective Synthesis of Chiral ansa-Metallocenes




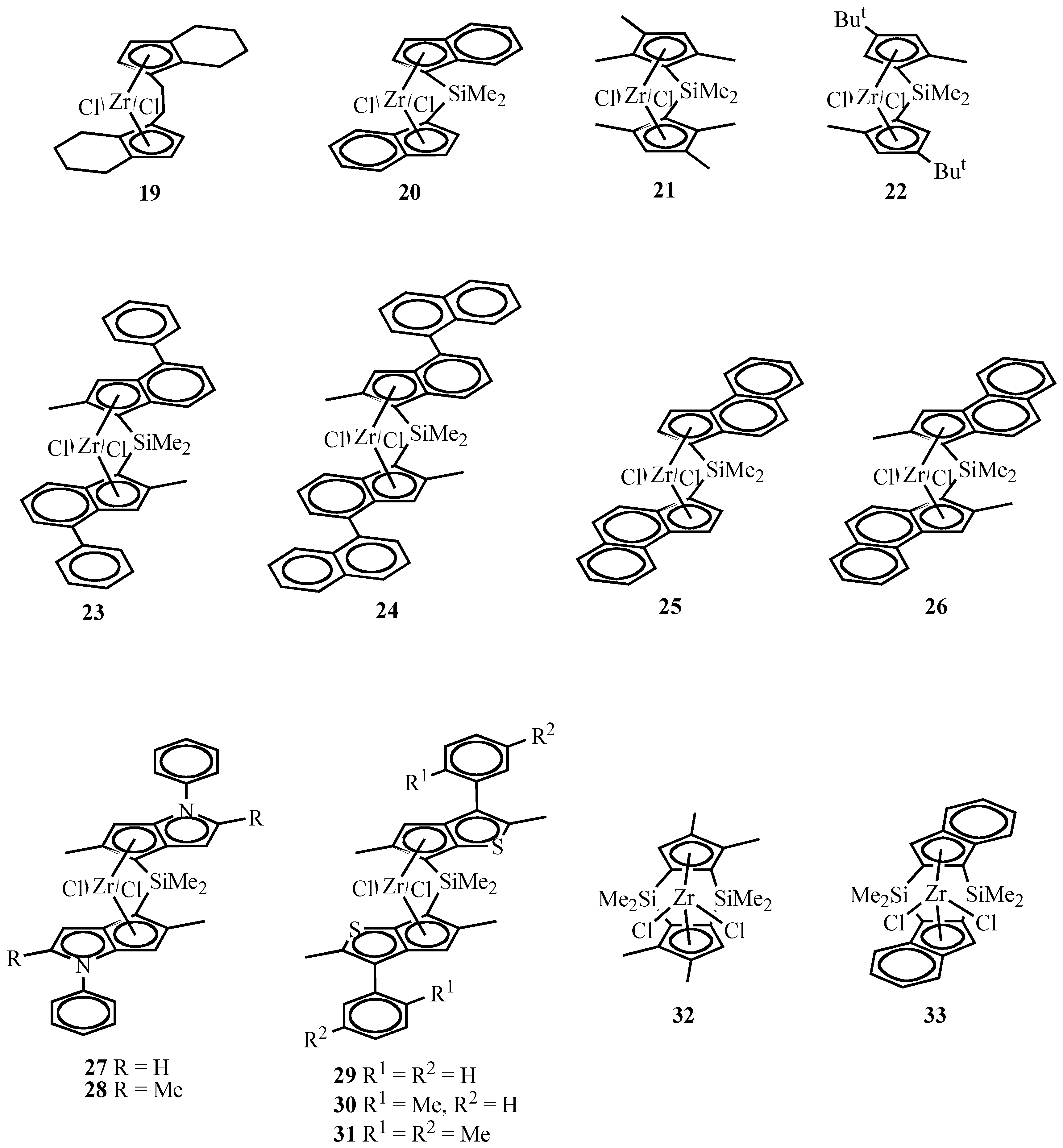
Isospecific Polymerization of Propylene by Chiral ansa-Type (Bridged) Metallocenes
| Catalyst | Temp (°C) | Activitya | Mn/103 | Mw/Mn | mmmm (%) | Tm (°C) | ref. |
| ansa-Metallocenes | |||||||
| (C2H4)(Ind)2ZrCl2 (2) | 60 | 1.375 | 6.3 | 1.9 | 63 | — | [58] |
| 30 | 7.0 | 35.9 b | 2.40 | 85.2 | 142.3 | [22] | |
| rac-(C2H4)(IndH4)2ZrCl2 (19) | 20 | 4.75 | 45 b | 1.9 | 95c | — | [18] |
| rac-Me2Si(Ind)2ZrCl2 (20) | 65 | 11.0 d | 44 b | 2.2 | 96.2 c | — | [21] |
| rac-Me2Si(2,3,5-Me3Cp)2ZrCl2 (21) | 30 | 5.3 | 134b | 1.99 | 97.7 | 162.0 | [22] |
| rac-Me2Si(3-But-5-MeCp)2ZrCl2 (22) | 50 | 0.505 | 9.19 b | 2.5 | 94 | 149 | [23] |
| rac-Me2Si(2-Me-4-PhInd)2ZrCl2 (23) | 70 | 755 | 729 | — | 95.2 | 157 | [24] |
| rac-Me2Si(2-Me-4-NaphInd) 2ZrCl2 (24) | 70 | 875 | 920 | — | 99.1 | 161 | [24] |
| rac-Me2Si(Benz[e]Ind)2 ZrCl2 (25) | 40 | 37.5 | 28 | 1.7 | 93.9 | 146.6 | [26] |
| rac-Me2Si(2-Me-Benz[e]Ind)2ZrCl2 (26) | 40 | 45.0 | 248 | 2.2 | 96.2 | 164.1 | [26] |
| rac-Me2Si(5-Me-1-Ph-4-Pyr[b]Cp)2ZrCl2 (27) | 70 | 443d | 145b | — | — | 146 | [27] |
| rac-Me2Si(2,5-Me2-1-Ph-4-Pyr[b]Cp)2ZrCl2 (28) | 70 | 324d | 198b | — | — | 155 | [27] |
| rac-Me2Si(2,5-Me2-3-Ph-6-Tp[b]Cp)2ZrCl2 (29) | 70 | 1953d | 445b | — | — | 156 | [27] |
| rac-Me2Si{2,5-Me2-3-(2-MeC6H4)-6-Tp[b]-Cp}2ZrCl2 (30) | 70 | 850d | 604b | — | — | 160 | [27] |
| rac-Me2Si{2,5-Me2-3-(2,5-Me2C6H3)-6-Tp[b] Cp}2ZrCl2 (31) | 70 | 479d | 795b | — | — | 160 | [27] |
| rac-(C2H4)(Ind)2HfCl2 (5) | 50 | 43.6 | 304b | 2.3 | 95–99 e | 136 | [29] |
| rac-(C2H4)(IndH4)2HfCl2 (34) | 50 | 9.1 | 150b | 2.2 | 95–99 e | 142 | [29] |
| rac-Me2Si(2,3,5-Me3Cp)2HfCl2 | 30 | 0.10 | 256 | 2.38 | 98.7 | 162.8 | [22] |
| (C2H4)(Ind)2TiCl2 (4) (56% rac 44% meso) | –60 | 0.018d | 96.6 | 1.59 | 54c | — | [16] |
| MeCH(Ind)(C5Me4)TiCl2 (35) | 50 | 0.167 | 66.6 | 1.9 | 40 | 51.2 66.0 | [32] |
| Unbridged Metallocenes | |||||||
| Cp2TiPh2 (37) | –60 | 0.055d | 195 | 1.8 | 53 | — | [16] |
| rac-(PhMeCHCp)2ZrCl2 (38) | –50 | 0.026d | 310f | — | 43 | — | [36] |
| rac-(CyMeCHCy)2ZrCl2 (39) | –50 | 0.003d | 86f | — | 51 | — | [36] |
| rac-{(9-BBN)CH2CH(Ph)Cp}2ZrCl2 (40) | –50 | 0.012d | 11f | — | 54 | — | [36] |
| rac-{(9-BBN)CH2CH(Cy)Cp}2ZrCl2 (41) | –50 | 0.005d | 10f | — | 75 | — | [36] |
| {2-(1-neoisomenthyl)Ind}2ZrCl2 (42) | –30 | 0.032d | 260f | — | 77 | — | [37] |
| {2-(3’-α-cholestanyl)Ind}2ZrCl2 (43) | –30 | 0.041d | 470 f | — | 80 | — | [38] |
| (1-MeFlu)2ZrCl2 (44) | 60 | 0.035d | 65b | — | 83 | 145 | [40] |
| b Mw value. | e mole % of units with the same relative configuration. |
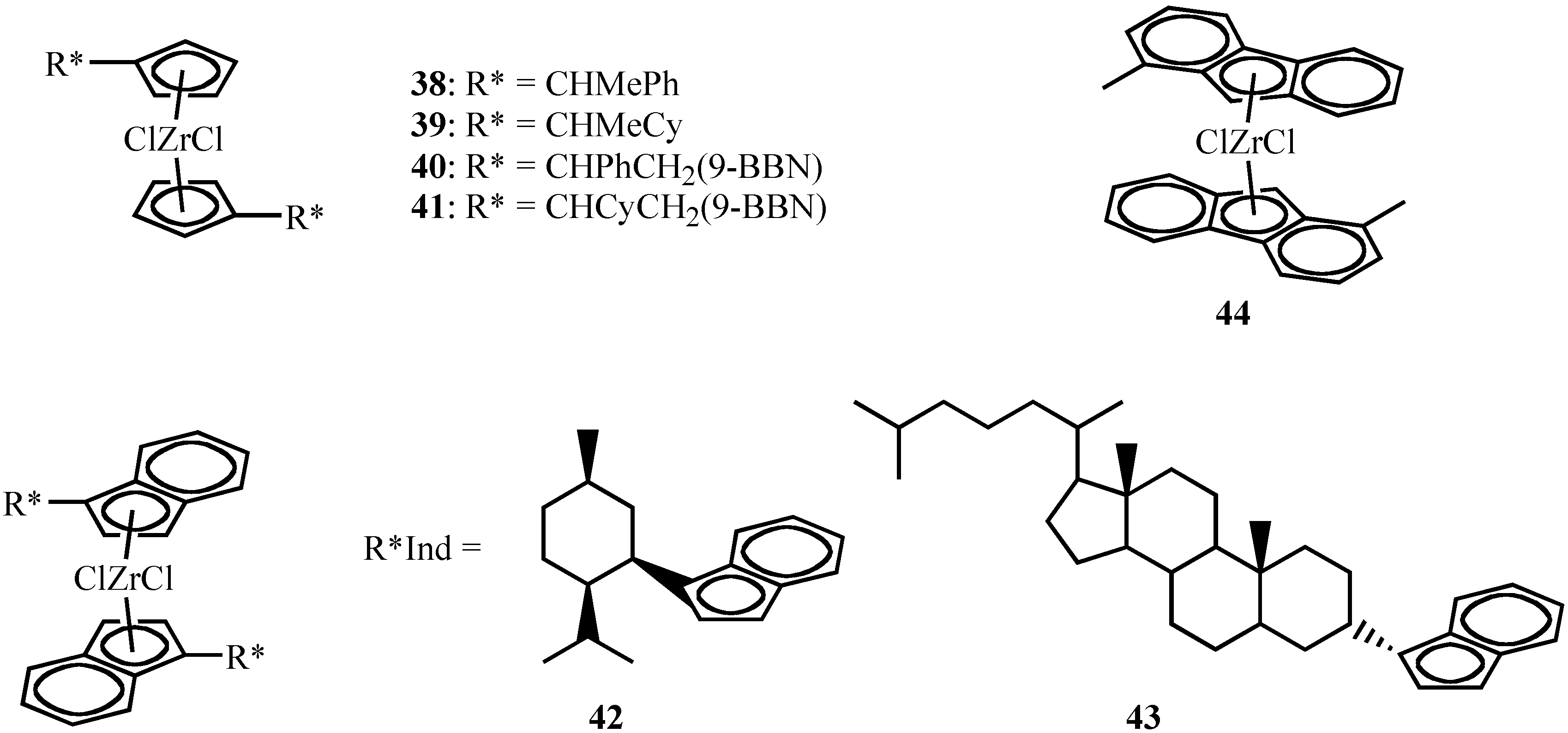
Isospecific Polymerization of Propylene by Unbridged Metallocenes
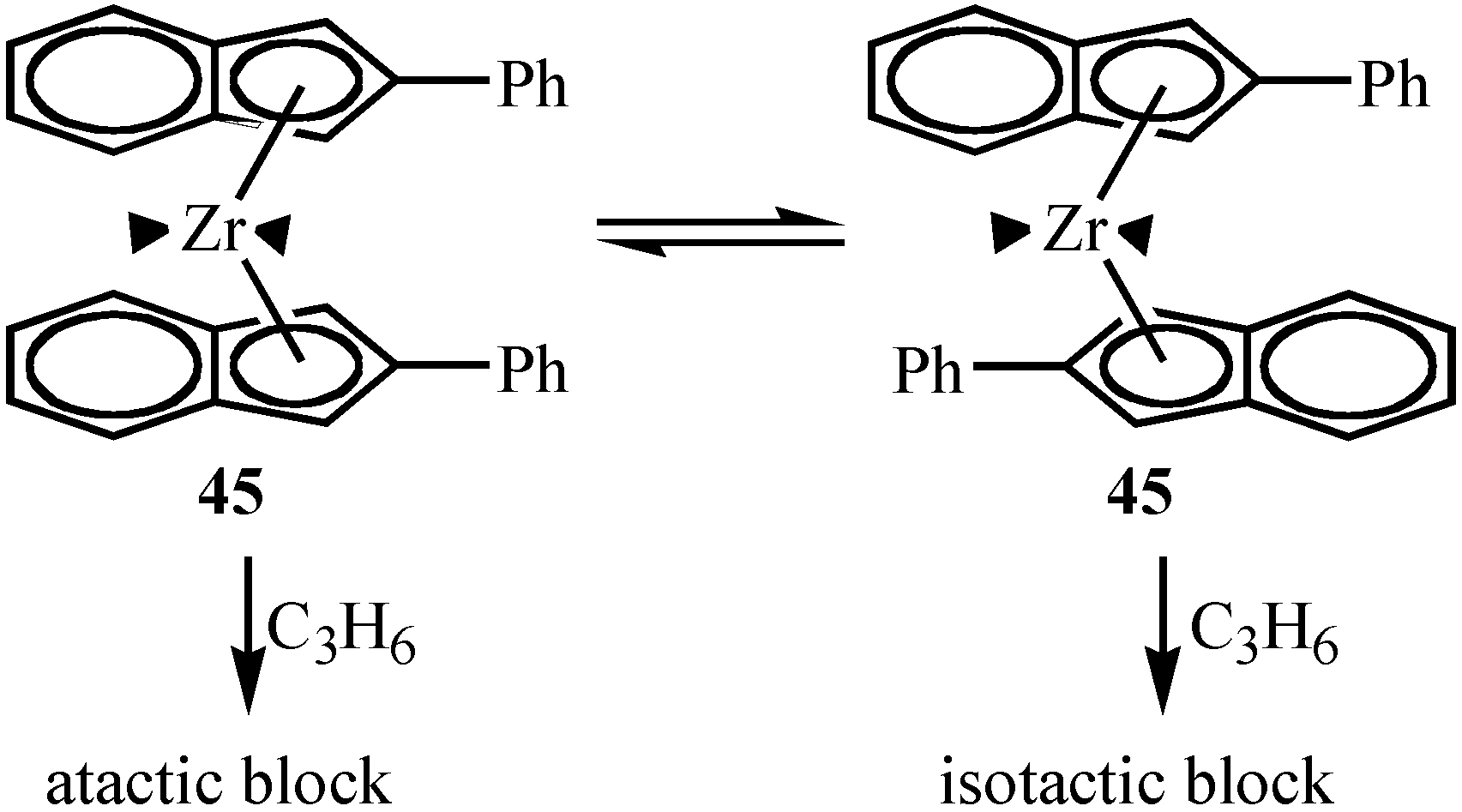
Asymmetric Polymerization by Enantiopure Chiral Metallocenes

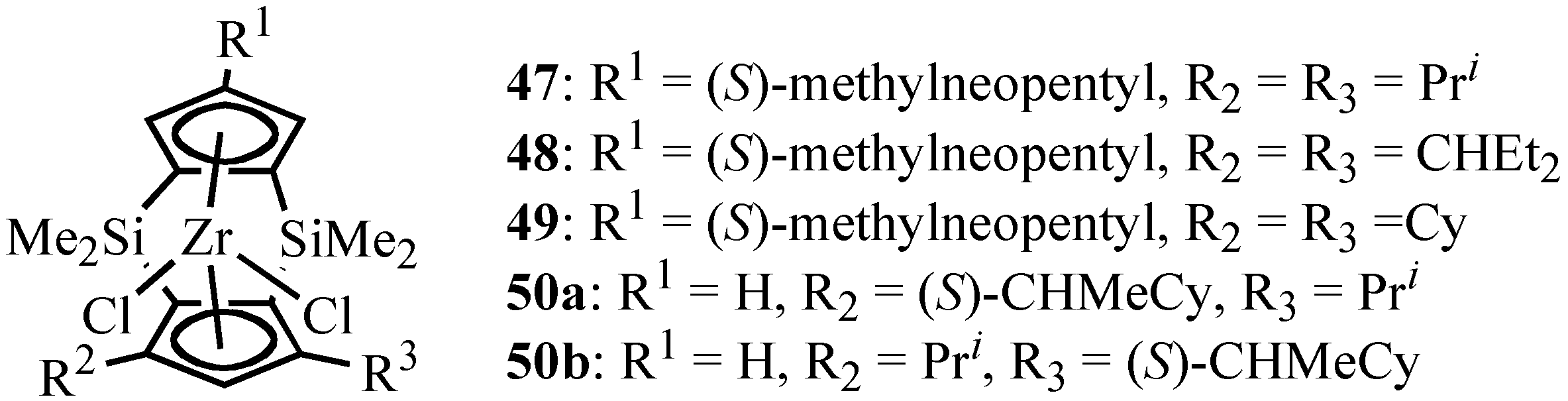
Isospecific Polymerization of Polar Monomers
| Catalyst (Initiator) | Temp (°C) | [MMA]0/[cat.] | Time (h) | Yield (%) | Mn/103 | Mw/Mn | mm (%) | mr (%) | rr (%) | ref. |
| rac-(C2H4)(Ind)2ZrMe2 (54) /[Ph3C][B(C6F5)4] /Zn(CH2CH2CH=CH2)2 | 0 | 1870 | 24 | 48 | 599 | 1.30 | 98.0 | 1.3 | 0.7 | [50] |
| rac-(C2H4)(IndH4)2ZrMe2 (55) /[Ph3C][B(C6F5)4] /Zn(CH2CH2CH=CH2)2 | 0 | 1870 | 24 | 64 | 339 | 1.25 | 95.5 | 3.3 | 1.2 | [50] |
| rac-Me2Si(Ind)2ZrMe2 (56) /[Ph3C][B(C6F5)4] /Zn(CH2CH2CH=CH2)2 | 0 | 1870 | 24 | 34 | 345 | 1.34 | 91.4 | 5.3 | 2.3 | [50] |
| rac-Me2Si(Ind)2ZrMe2 (56) /B(C6F4-2-C6F5)3 | 0 | 935 | 5.5 | 100 | — | — | 93.0 | 4.8 | 2.2 | [51] |
| rac-(C2H4)(Ind)2ZrMe2 (54)/B(C6F5)3 | r.t. | 200 | 1 | 98 | 48.7 | 1.20 | 95 | 4 | 1 | [52] |
| rac-Me2Si(Ind)2ZrMe2 (56)/Al(C6F5)3 | 0 | 200 | 4 | 100 | 73.9 | 1.32 | 4.6 | 34.6 | 60.8 | [53] |
| rac-(C2H4)(Ind)2ZrMe{OC(OPri)=CMe2} (57)/B(C6F5)3 | r.t. | 400 | 1/6 | >99 | 48.6 | 1.03 | 96.7 | 2.2 | 1.1 | [55] |
| [rac-(C2H4)(Ind)2Zr{OC(OPri)=CMe2}]-[MeB(C6F5)3] (58) | r.t. | 600 | 1/6 | >99 | 75.5 | 1.03 | 95.3 | 3.2 | 1.5 | [55] |
| rac-(C2H4)(Ind)2Zr+Me-OCPriOAl–(C6F5)3 (59) | r.t. | 400 | 20 | 90 | 51.9 | 1.93 | 6.6 | 34.2 | 59.2 | [55] |
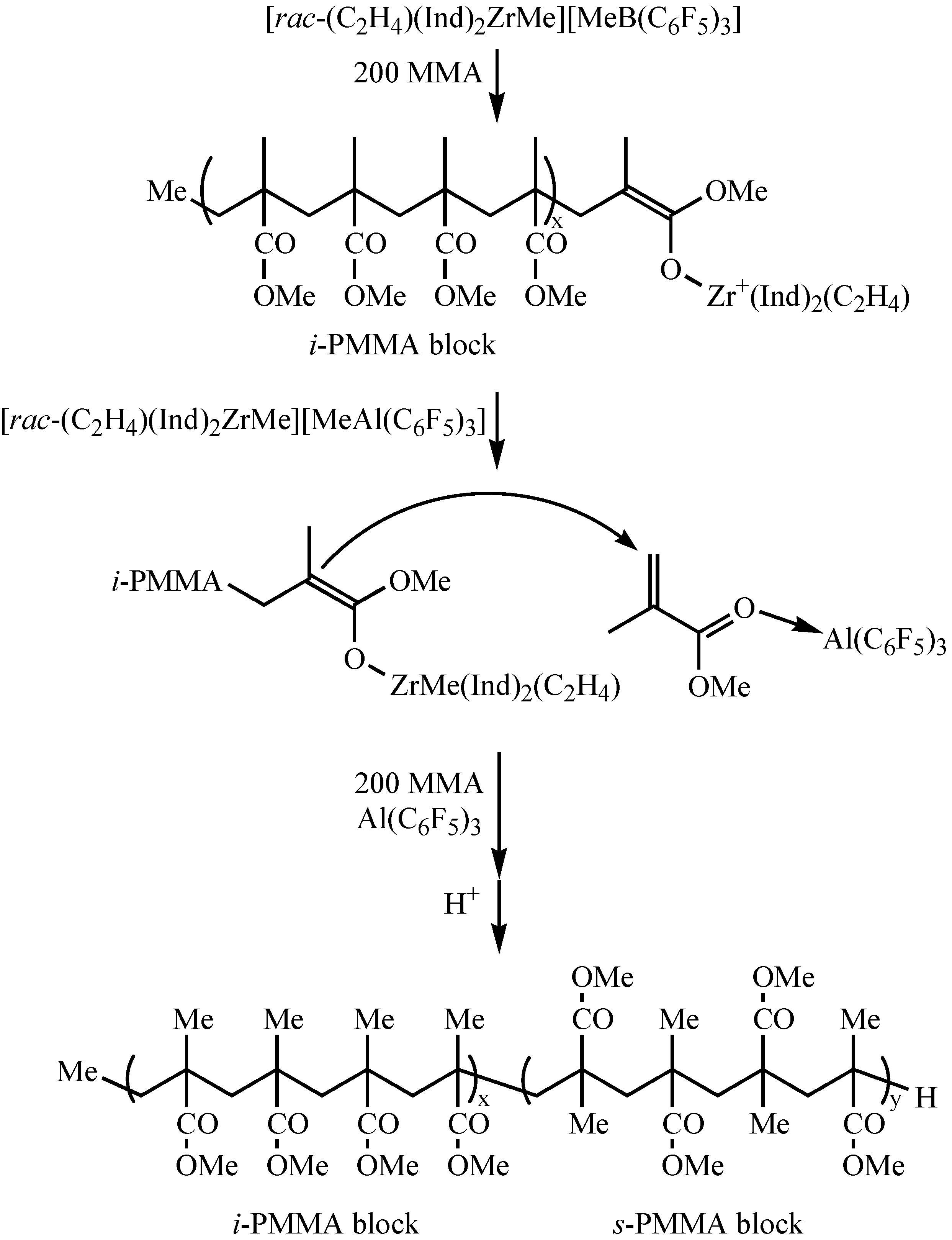
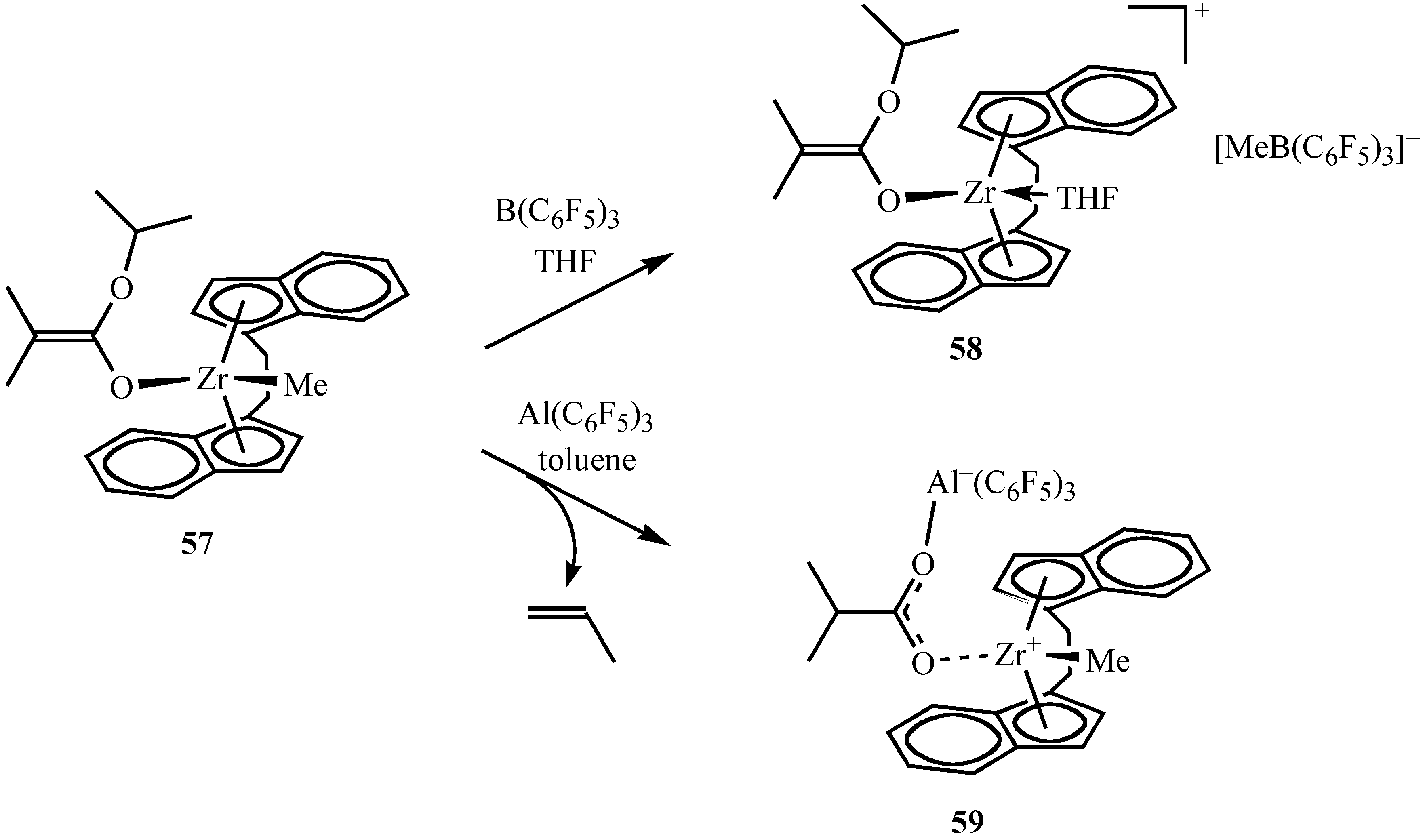
Conclusions
References
- Scheirs, J.; Kaminsky, W. Metallocene-Based Polyolefins; John Wiley & Suns Ltd: West Sussex, 2000. [Google Scholar]
- Mashima, K.; Nakayama, Y.; Nakamura, A. Adv. Polym. Sci. 1997, 133, 1.
- Britovsek, G. J. P.; Gibson, V. C.; Wass, D. F. Angew. Chem. Int. Ed. 1999, 38, 428. [CrossRef]
- Wild, F. R. W. P.; Zsolnai, L.; Huttner, G.; Brintzinger, H. H. J. Organomet. Chem. 1982, 232, 233.
- Wild, F. R. W. P.; Wasiucionek, M.; Huttner, G.; Brintzinger, H. H. J. Organomet. Chem. 1985, 288, 63.
- Grossman, R. B.; Doyle, R. A.; Buchwald, S. L. Organometallics 1991, 10, 1501.
- Lee, I.-M.; Gauthier, W. J.; Ball, J. M.; Iyengar, B.; Collins, S. Organometallics 1992, 11, 2115.
- Erickson, M. S.; Fronczek, F. R.; McLaughlin, M. L. J. Organomet. Chem. 1991, 415, 75.
- Damrau, H.-R. H.; Royo, E.; Obert, S.; Schaper, F.; Weeber, A.; Brintzinger, H.-H. Organometallics 2001, 20, 5258.
- Diamond, G. M.; Jordan, R. F.; Petersen, J. L. J. Am. Chem. Soc. 1996, 118, 8024.
- Diamond, G. M.; Jordan, R. F.; Petersen, J. L. Organometallics 1996, 16, 4030.
- Christopher, J. N.; Diamond, G. M.; Jordan, R. F.; Petersen, J. L. Organometallics 1996, 15, 4038.
- Diamond, G. M.; Jordan, R. F.; Petersen, J. L. Organometallics 1996, 15, 4045.
- Zhang, X.; Zhu, Q.; Guzei, I. A.; Jordan, R. F. J. Am. Chem. Soc. 2000, 122, 8093.
- Chen, E. Y.-X.; Richard, E.; Campbell, J.; Devore, D. D.; Green, D. P.; Link, B.; Soto, J.; Wilson, D. R.; Abboud, K. A. J. Am. Chem. Soc. 2004, 126, 42.
- Ewen, J. A. J. Am. Chem. Soc. 1984, 106, 6355.
- Rieger, B.; Mu, X.; Mallin, D. T.; Rausch, M. D.; Chien, J. C. W. Macromolecules 1990, 23, 3559.
- Kaminsky, W.; Kulper, K.; Brintzinger, H. H.; Wild, F. R. W. P. Angew. Chem., Int. Ed. Engl. 1985, 24, 507. [CrossRef]
- Grassi, A.; Zambelli, A.; Resconi, L.; Albizzati, E.; Mazzocchi, R. Macromolecules 1988, 21, 617.
- Longo, P.; Grassi, A.; Pellecchia, C.; Zambelli, A. Macromolecules 1987, 20, 1015.
- Herrmann, W. A.; Rohrmann, J. Angew. Chem., Int. Ed. Engl. 1989, 28, 1511. [CrossRef]
- Mise, T.; Miya, S.; Yamazaki, H. Chem. Lett. 1989, 1853.
- Röll, W.; Brinztinger, H.-H.; Rieger, B.; Zolk, R. Angew. Chem., Int. Ed. Engl. 1990, 29, 279. [CrossRef]
- Spaleck, W.; Küber, F.; Winter, A.; Rohrmann, J.; Bachmann, B.; Antberg, M.; Dolle, V.; Paulus, E. F. Organometallics 1994, 13, 954.
- Stehling, U.; Diebold, J.; Röll, W.; Brintzinger, H. H. Organometallics 1994, 13, 964.
- Jüngling, S.; Mülhaupt, R.; Stehling, U.; Brintzinger, H.-H.; Fischer, D.; Langhauser, F. J. Polym. Sci., Part A: Polym. Chem. 1995, 33, 1305.
- Ewen, J. A.; Elder, M. J.; Jones, R. L.; Rheingold, A. L.; Liable-Sands, L. M.; Sommer, R. D. J. Am. Chem. Soc. 2001, 123, 4763.
- Mengele, W.; Diebold, J.; Troll, C.; Röll, W.; Brintzinger, H.-H. Organometallics 1993, 12, 1931.
- Ewen, J. A.; Haspeslagh, L.; Atwood, J. L.; Zhang, H. J. Am. Chem. Soc. 1987, 109, 6544.
- Farina, M.; Silvestro, G. D.; Sozzani, P. Macromolecules 1993, 26, 946.
- Ewen, J. A. Macromol. Symp. 1995, 89, 181. [CrossRef]
- Mallin, D. T.; Rausch, M. D.; Lin, Y.-G.; Dong, S.; Chien, J. C. W. J. Am. Chem. Soc. 1990, 112, 2030.
- Chien, J. C. W.; Llinas, G. H.; Rausch, M. D.; Lin, G.-Y.; Winter, H. H.; Atwood, J. L.; Bott, S. G. J. Am. Chem. Soc. 1991, 113, 8569.
- Llinas, G. H.; Dong, S.-H.; Mallin, D. T.; Rausch, M. D.; Lin, Y.-G.; Winter, H. H.; Chien, J. C. W. Macromolecules 1992, 25, 1242.
- Erker, G.; Nolte, R.; Tsay, Y.-H.; Krüger, C. Angew. Chem., Int. Ed. Engl. 1989, 28, 628. [CrossRef]
- Erker, G.; Nolte, R.; Aul, R.; Wilker, S.; Krüger, C.; Noe, R. J. Am. Chem. Soc. 1991, 113, 7594.
- Erker, G.; Aulbach, M.; Knickmeier, M.; Wingbermühle, D.; Krüger, C.; Nolte, M.; Werner, S. J. Am. Chem. Soc. 1993, 115, 4590.
- Erker, G.; Temme, B. J. Am. Chem. Soc. 1992, 114, 4004.
- Erker, G. Pure and Appl. Chem. 1992, 64, 393.
- Razavi, A.; Atwood, J. L. J. Am. Chem. Soc. 1993, 115, 7529.
- Coates, C. W.; Waymouth, R. M. Science 1995, 267, 217.
- Coates, G. W.; Waymouth, R. M. J. Am. Chem. Soc. 1993, 115, 91.
- Waymouth, R.; Pino, P. J. Am. Chem. Soc. 1990, 112, 4911.
- Pino, P. J. Organomet. Chem. 1990, 370, 1.
- Baar, C. R.; Levy, C. J.; Min, E. Y.-J.; Henling, L. M.; Day, M. W.; Bercaw, J. E. J. Am. Chem. Soc. 2004, 126, 8216.
- Collins, S.; Ward, D. G. J. Am. Chem. Soc. 1992, 114, 5460.
- Li, Y.; Ward, D. G.; Reddy, S. S.; Collins, S. Macromolecules 1997, 30, 1875.
- Collins, S.; Ward, D. G.; Suddaby, K. H. Macromolecules 1994, 2, 24.
- Soga, K.; Deng, H.; Yano, T.; Shiono, T. Macromolecules 1994, 27, 7938.
- Deng, H.; Shiono, T.; Soga, K. Macromolecules 1995, 28, 3067.
- Chen, Y.-X. E.; Metz, M. V.; Li, L.; Stern, C. L.; Marks, T. J. J. Am. Chem. Soc. 1998, 120, 6287.
- Cameron, P. A.; Gibson, V. C.; Graham, A. J. Macromolecules 2000, 33, 4329.
- Bolig, A. D.; Chen, E. Y.-X. J. Am. Chem. Soc. 2001, 123, 7943.
- Bolig, A. D.; Chen, E. Y.-X. J. Am. Chem. Soc. 2002, 124, 5612.
- Bolig, A. D.; Chen, E. Y.-X. J. Am. Chem. Soc. 2004, 126, 4897.
- Strauch, J. W.; Faure, J.-L.; Bredeau, S.; Wang, C.; Kehr, G.; Frohlich, R.; Luftmann, H.; Erker, G. J. Am. Chem. Soc. 2004, 126, 2089.
- Mariott, W. R.; Chen, E. Y.-X. Macromolecules 2004, 37, 4741.
- Bochmann, M.; Lancaster, S. J. Organometallics 1993, 12, 633.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Nakayama, Y.; Shiono, T. Developments of Chiral Metallocenes as Polymerization Catalysts. Molecules 2005, 10, 620-633. https://doi.org/10.3390/10060620
Nakayama Y, Shiono T. Developments of Chiral Metallocenes as Polymerization Catalysts. Molecules. 2005; 10(6):620-633. https://doi.org/10.3390/10060620
Chicago/Turabian StyleNakayama, Yuushou, and Takeshi Shiono. 2005. "Developments of Chiral Metallocenes as Polymerization Catalysts" Molecules 10, no. 6: 620-633. https://doi.org/10.3390/10060620
APA StyleNakayama, Y., & Shiono, T. (2005). Developments of Chiral Metallocenes as Polymerization Catalysts. Molecules, 10(6), 620-633. https://doi.org/10.3390/10060620





