Vaccines 2021, 9(7), 699; https://doi.org/10.3390/vaccines9070699 - 25 Jun 2021
Cited by 5 | Viewed by 2873
Abstract
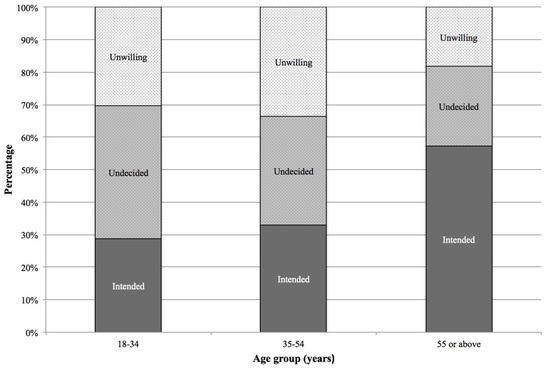
Nowadays, mumps is re-emerging in highly vaccinated populations. Waning of vaccine-induced immunity plays a role, but antigenic differences between vaccine and mumps outbreak strains could also contribute to reduced vaccine effectiveness. CD8+ T cells play a critical role in immunity to viruses.
[...] Read more.
Nowadays, mumps is re-emerging in highly vaccinated populations. Waning of vaccine-induced immunity plays a role, but antigenic differences between vaccine and mumps outbreak strains could also contribute to reduced vaccine effectiveness. CD8+ T cells play a critical role in immunity to viruses. However, limited data are available about sequence variability in CD8+ T cell epitope regions of mumps virus (MuV) proteins. Recently, the first set of naturally presented human leukocyte antigen Class I (HLA-I) epitopes of MuV was identified by us. In the present study, sequences of 40 CD8+ T cell epitope candidates, including previously and newly identified, obtained from Jeryl–Lynn mumps vaccine strains were compared with genomes from 462 circulating MuV strains. In 31 epitope candidates (78%) amino acid differences were detected, and in 17 (43%) of the epitope candidates the corresponding sequences in wild-type strains had reduced predicted HLA-I-binding compared to the vaccine strains. These findings suggest that vaccinated persons may have reduced T cell immunity to circulating mumps viruses due to antigenic differences.
Full article
(This article belongs to the Section Vaccines against Tropical and other Infectious Diseases)
►
Show Figures