1
Department of Experimental and Clinical Pharmacology, Medical University of Warsaw, Center for Preclinical Research and Technology CEPT, 02-097 Warsaw, Poland
2
Rheumatology Division, Hospital das Clinicas HCFMUSP, Universidade de Sao Paulo, Sao Paulo, SP 01246-903, Brazil
3
Division of Cardiology, Department of Medical and Surgical Sciences, “Magna Graecia” University, 88100 Catanzaro, Italy
4
URT-CNR, Department of Medicine, Consiglio Nazionale delle Ricerche of IFC, Viale Europa S/N, 88100 Catanzaro, Italy
5
2nd Department of Neurology, Institute of Psychiatry and Neurology, 02-957 Warsaw, Poland
†
These authors contributed equally to this work.
Cells 2018, 7(12), 249; https://doi.org/10.3390/cells7120249 - 6 Dec 2018
Cited by 160 | Viewed by 10394
Abstract
Stroke is the second-most common cause of death worldwide. The pathophysiology of ischemic stroke (IS) is related to inflammation, atherosclerosis, blood coagulation, and platelet activation. MicroRNAs (miRNAs) play important roles in physiological and pathological processes of neurodegenerative diseases and progression of certain neurological
[...] Read more.
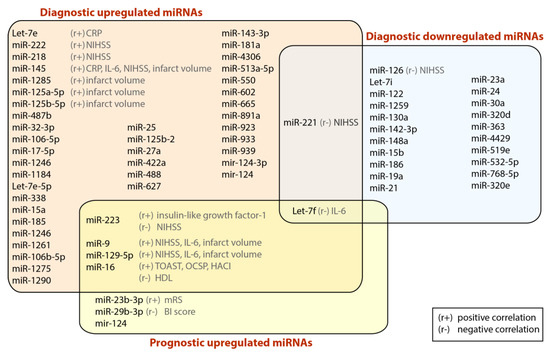
Stroke is the second-most common cause of death worldwide. The pathophysiology of ischemic stroke (IS) is related to inflammation, atherosclerosis, blood coagulation, and platelet activation. MicroRNAs (miRNAs) play important roles in physiological and pathological processes of neurodegenerative diseases and progression of certain neurological diseases, such as IS. Several different miRNAs, and their target genes, are recognized to be involved in the pathophysiology of IS. The capacity of miRNAs to simultaneously regulate several target genes underlies their unique value as diagnostic and prognostic markers in IS. In this review, we focus on the role of miRNAs as diagnostic and prognostic biomarkers in IS. We discuss the most common and reliable detection methods available and promising tests currently under development. We also present original results from bioinformatic analyses of published results, identifying the ten most significant genes (HMGB1, YWHAZ, PIK3R1, STAT3, MAPK1, CBX5, CAPZB, THBS1, TNFRSF10B, RCOR1) associated with inflammation, blood coagulation, and platelet activation and targeted by miRNAs in IS. Additionally, we created miRNA-gene target interaction networks based on Gene Ontology (GO) information derived from publicly available databases. Among our most interesting findings, miR-19a-3p is the most widely modulated miRNA across all selected ontologies and might be proposed as novel biomarker in IS to be tested in future studies.
Full article
(This article belongs to the Special Issue Regulatory microRNA)
▼
Show Figures