Cancers 2021, 13(12), 2905; https://doi.org/10.3390/cancers13122905 - 10 Jun 2021
Cited by 12 | Viewed by 6670
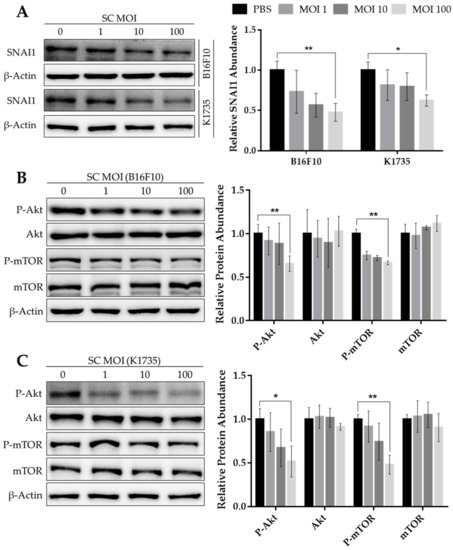
Abstract
Patients with cancer, both hematologic and solid malignancies, are at increased risk for thrombosis and thromboembolism. In addition to general risk factors such as immobility and major surgery, shared by non-cancer patients, cancer patients are exposed to specific thrombotic risk factors. These include,
[...] Read more.
Patients with cancer, both hematologic and solid malignancies, are at increased risk for thrombosis and thromboembolism. In addition to general risk factors such as immobility and major surgery, shared by non-cancer patients, cancer patients are exposed to specific thrombotic risk factors. These include, among other factors, cancer-induced hypercoagulation, and chemotherapy-mediated endothelial dysfunction as well as tumor-cell-derived microparticles. After an episode of thrombosis in a cancer patient, secondary thromboprophylaxis to prevent recurrent thromboembolism has long been established and is typically continued as long as the cancer is active or actively treated. On the other hand, primary prophylaxis, even though firmly established in hospitalized cancer patients, has only recently been studied in ambulatory patients. This recent change is mostly due to the emergence of direct oral anticoagulants (DOACs). DOACs have a shorter half-life than vitamin K antagonists (VKA), and they overcome the need for parenteral application, the latter of which is associated with low-molecular-weight heparins (LMWH) and can be difficult for the patient to endure in the long term. Here, first, we discuss the clinical trials of primary thromboprophylaxis in the population of cancer patients in general, including the use of VKA, LMWH, and DOACs, and the potential drug interactions with pre-existing medications that need to be taken into account. Second, we focus on special situations in cancer patients where primary prophylactic anticoagulation should be considered, including myeloma, major surgery, indwelling catheters, or immobilization, concomitant diseases such as renal insufficiency, liver disease, or thrombophilia, as well as situations with a high bleeding risk, particularly thrombocytopenia, and specific drugs that may require primary thromboprophylaxis. We provide a novel algorithm intended to aid specialists but also family practitioners and nurses who care for cancer patients in the decision process of primary thromboprophylaxis in the individual patient.
Full article
(This article belongs to the Section Cancer Therapy)
►
Show Figures