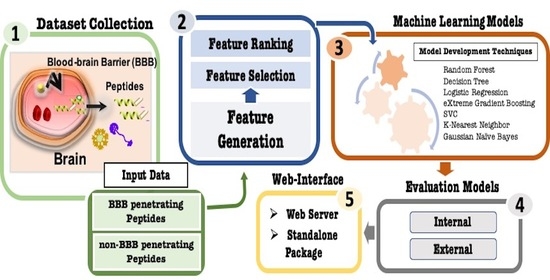
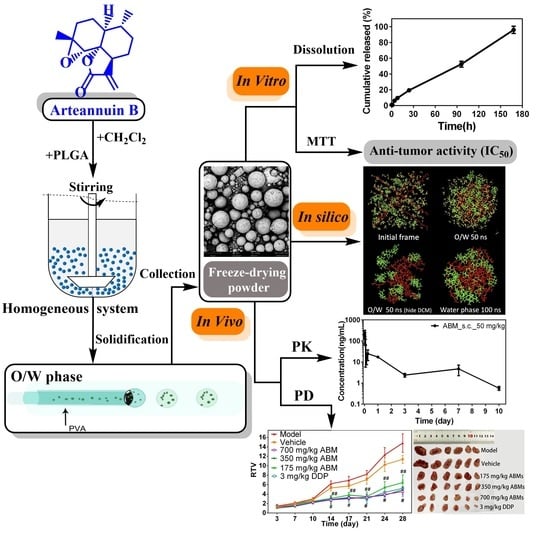
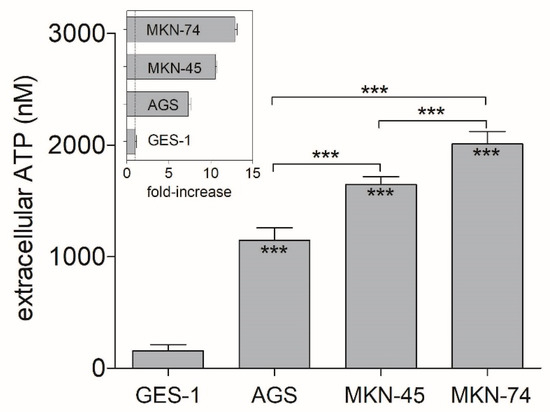
Pharmaceutics 2021, 13(8), 1247; https://doi.org/10.3390/pharmaceutics13081247 - 12 Aug 2021
Cited by 6 | Viewed by 3278
Abstract
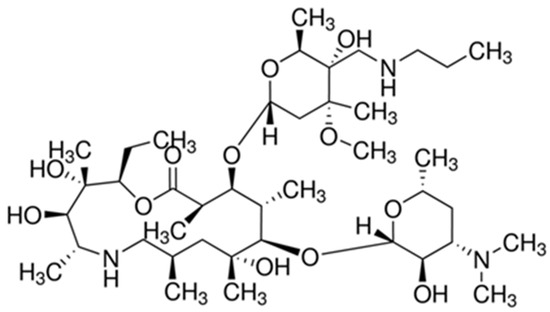
The present study was conducted to evaluate the analgesic potential of the new triamilide macrolide antibiotic, tulathromycin, at 20 and 40 mg/kg of body weight (BW), subcutaneously against acute pain in mice. Acute pain was induced either chemically (using acetic acid-induced writhing and
[...] Read more.
The present study was conducted to evaluate the analgesic potential of the new triamilide macrolide antibiotic, tulathromycin, at 20 and 40 mg/kg of body weight (BW), subcutaneously against acute pain in mice. Acute pain was induced either chemically (using acetic acid-induced writhing and formalin-induced pain tests) or thermally (using hot-plate, and tail-flick tests). In the acetic acid-induced writhing test, tulathromycin induced a dose-dependent and significant decrease in the number of writhes compared with the control group. In the late phase of the formalin test, a significant decline in hind paw licking time compared with the control group was observed. In the hot-plate and tail-flick tests, tulathromycin caused a dose-dependent and significant prolongation of latency of nociceptive response to heat stimuli, compared with the control group. These findings may indicate that tulathromycin possesses significant peripheral and central analgesic potentials that may be valuable in symptomatic relief of pain, in addition to its well-established antibacterial effect.
Full article
(This article belongs to the Special Issue Specific Drug Disposition in Veterinary Medicine)
►
Show Figures