1
Department of Chemistry, University of Colorado, Boulder, CO 80309, USA
2
Renewable and Sustainable Energy Institute, Boulder, CO 80309, USA
Energies 2020, 13(7), 1722; https://doi.org/10.3390/en13071722 - 4 Apr 2020
Cited by 2 | Viewed by 3862
Abstract
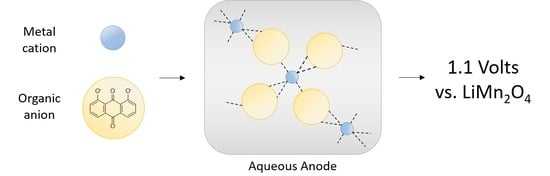
The need for expanded energy storage motivates material development for scalable aqueous secondary batteries. The combination of transition metals with redox-active organics represents a new approach to functional material design. Here, we detail the synthesis of titanium(IV) 1,8-dihydroxyanthraquinone (Ti(1,8-DHAQ)2) as a
[...] Read more.
The need for expanded energy storage motivates material development for scalable aqueous secondary batteries. The combination of transition metals with redox-active organics represents a new approach to functional material design. Here, we detail the synthesis of titanium(IV) 1,8-dihydroxyanthraquinone (Ti(1,8-DHAQ)2) as a novel redox-active material and demonstrate its use as a negative electrode in an aqueous battery. This one-pot synthesis results in amorphous micron-scale particles with titanium binding directly to the carbonyl feature as evidenced by scanning electron microscopy and infrared spectroscopy. When assembled in a coin cell with a lithium manganese oxide positive electrode, the active material can be electrochemically cycled with a charge density of 40 mAh/g at 1.1 V. This represents a new method of creating simple and scalable electrodes using metal-organic materials for versatile energy storage applications.
Full article
(This article belongs to the Special Issue Advanced Battery Technologies for Energy Storage Devices)
▼
Show Figures