Active Immunity Induced by Passive IgG Post-Exposure Protection against Ricin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Goat IgG and F(ab’)2 Preparation
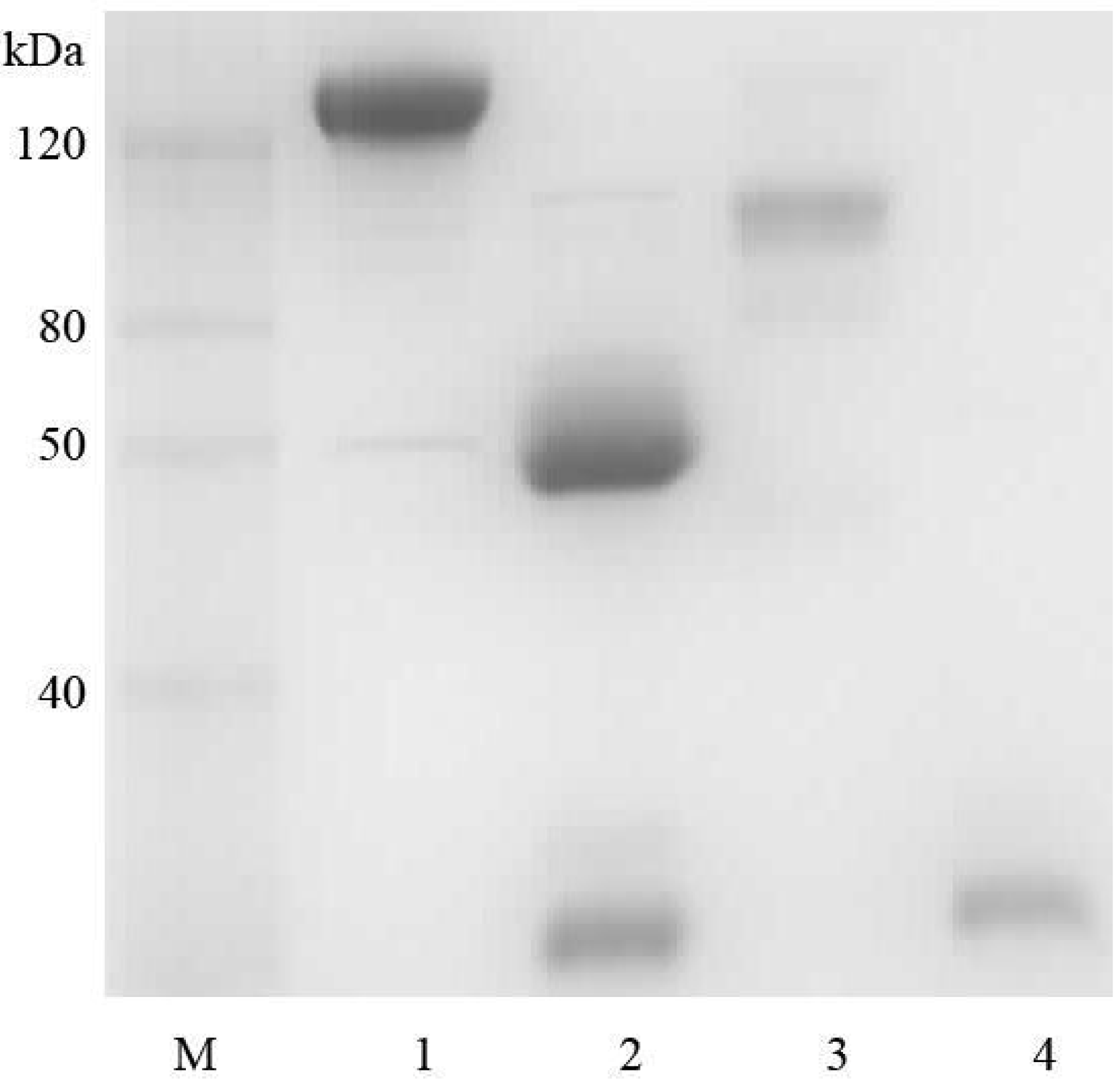
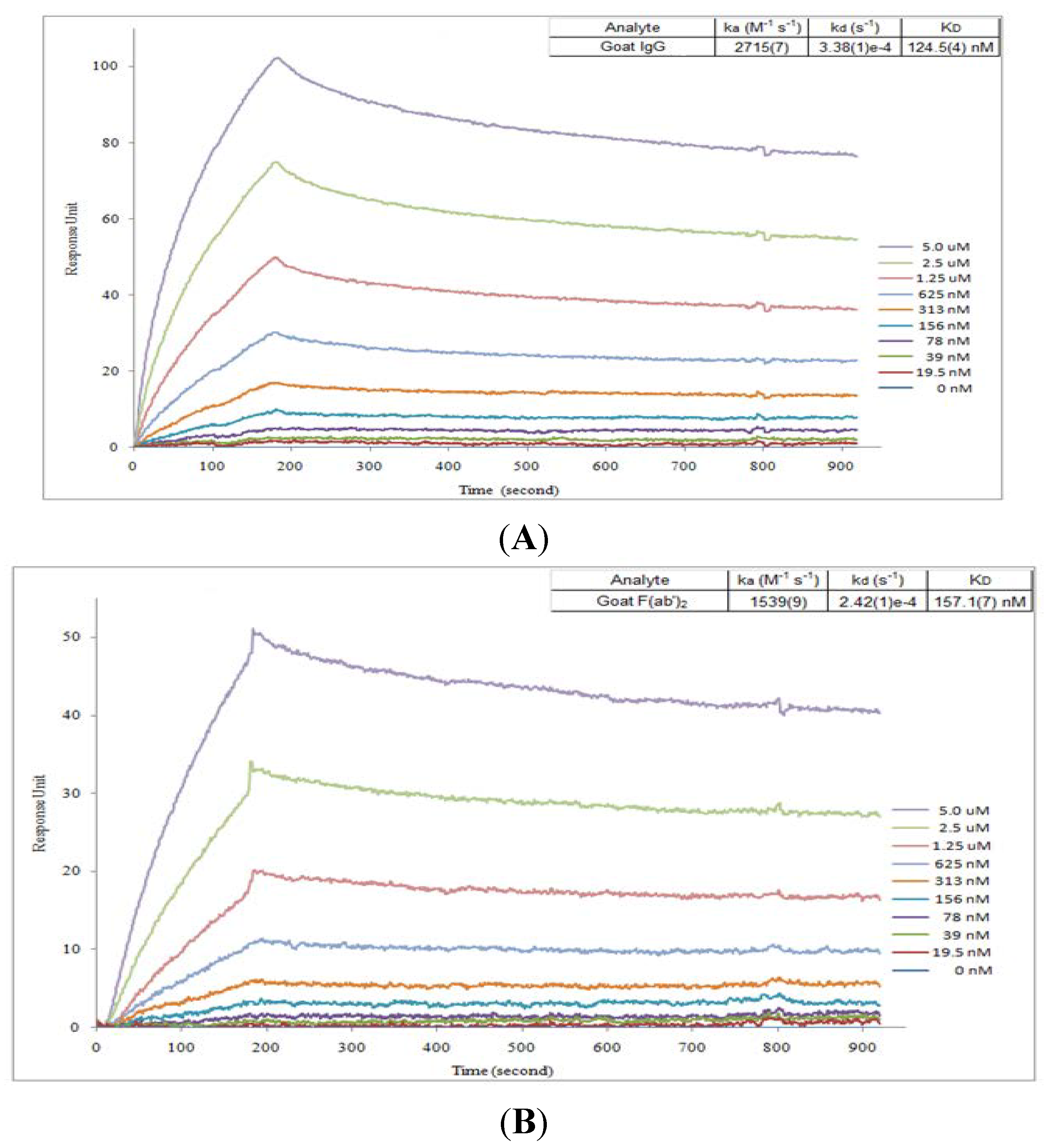
2.2. Affinity Assay for Goat IgG and F(ab’)2
2.3. Neutralization Assay for Goat IgG and F(ab’)2 in Vitro
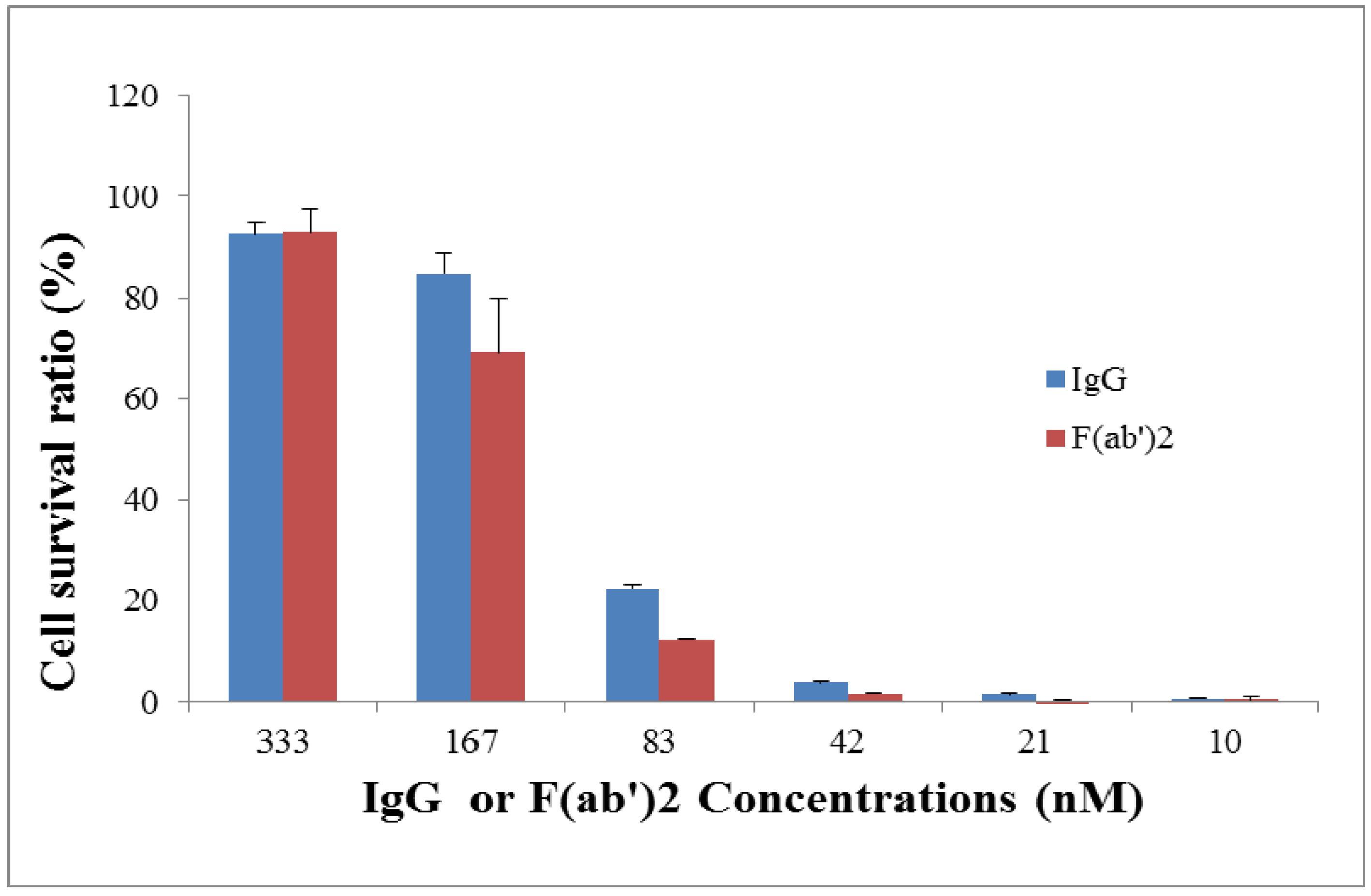
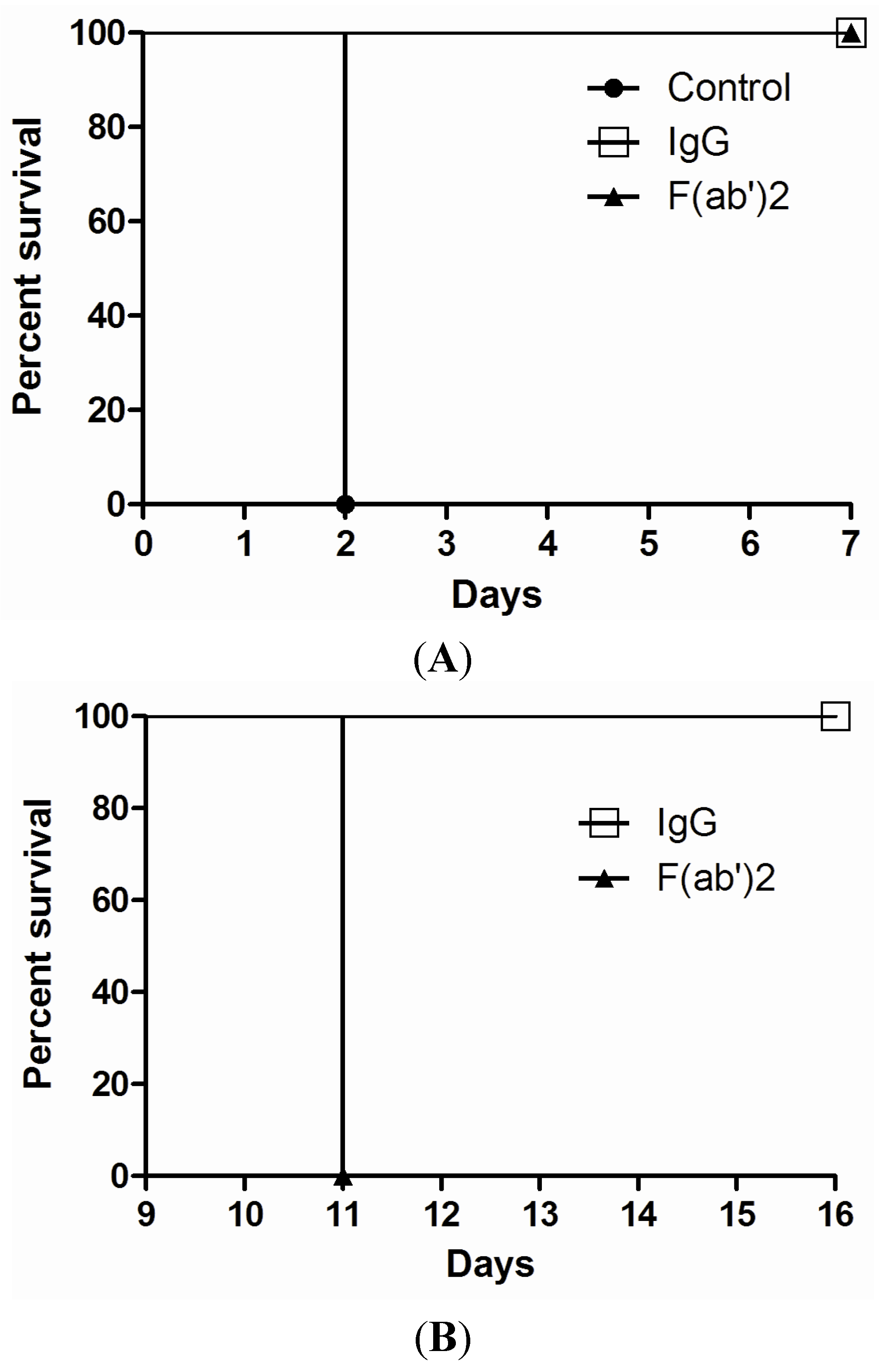
2.4. Efficacy of Goat IgG and F(ab’)2 in Vivo
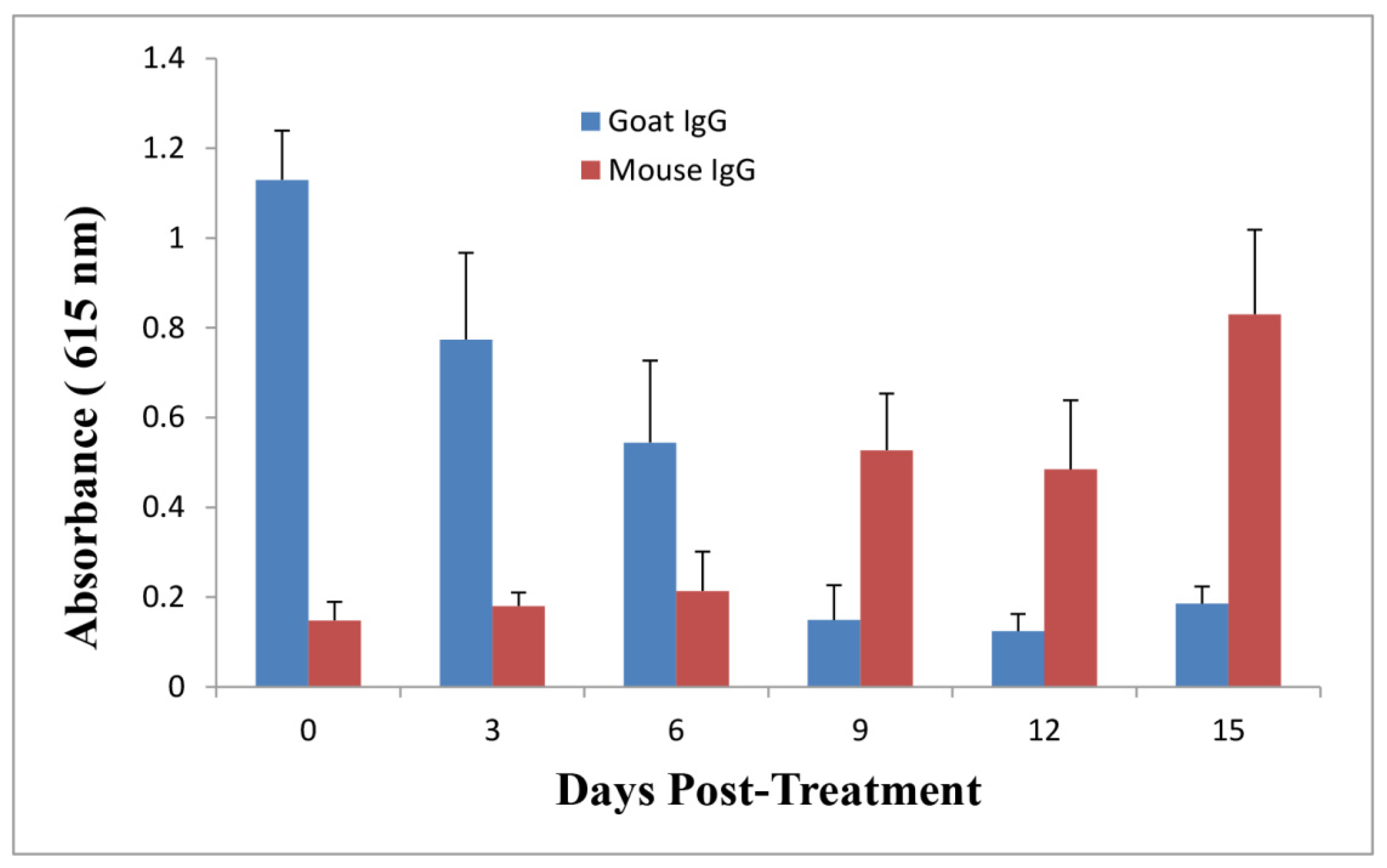
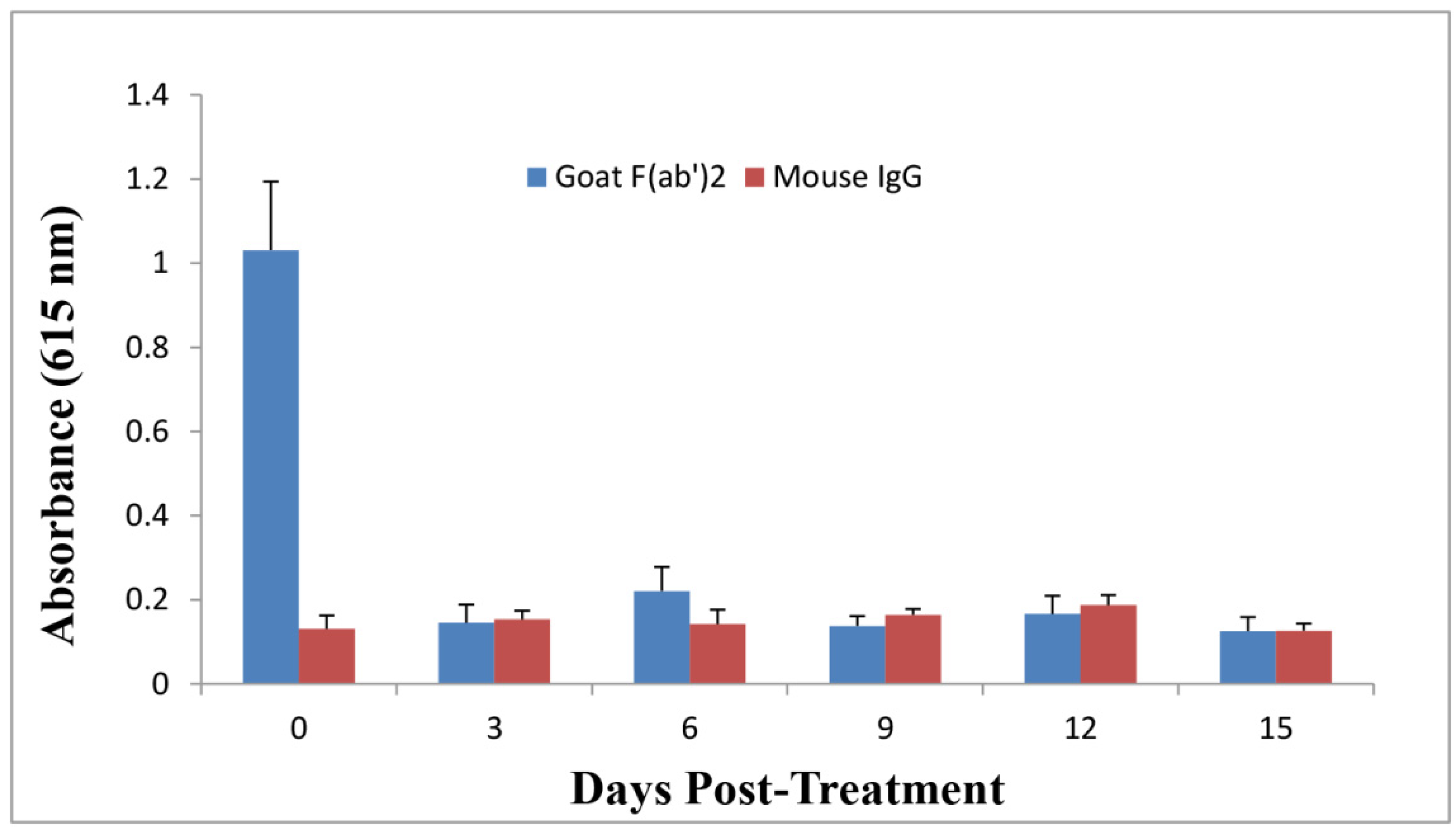
2.5. Anti-Ricin Antibody Levels in Mice
3. Experimental Section
3.1. Preparation of Goat IgG and F(ab’)2
3.2. Preparation of Ricin Stock
3.3. Affinity Analysis
3.4. In Vitro Neutralization Assay
3.5. In Vivo Protection Assay
3.6. ELISA
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zeitlin, L.; Cone, R.A.; Moench, T.R.; Whaley, K.J. Preventing infectious disease with passive immunization. Microbes Infect. 2000, 2, 701–708. [Google Scholar] [CrossRef]
- Little, S.F.; Ivins, B.E.; Fellows, P.F.; Friedlander, A.M. Passive protection by polyclonal antibodies against bacillus anthracis infection in guinea pigs. Infect. Immun. 1997, 65, 5171–5175. [Google Scholar]
- Casadevall, A.; Dadachova, E.; Pirofski, L.A. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004, 2, 695–703. [Google Scholar] [CrossRef]
- Casadevall, A. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg. Infect. Dis. 2002, 8, 833–841. [Google Scholar] [CrossRef]
- Casadevall, A. Passive antibody therapies: Progress and continuing challenges. Clin. Immunol. 1999, 93, 5–15. [Google Scholar] [CrossRef]
- Fda Approves Raxibacumab to Treat Inhalation Anthrax. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm332341.htm (accessed on 10 Jan., 2014).
- Heard, K.; O’Malley, G.F.; Dart, R.C. Antivenom therapy in the americas. Drugs 1999, 58, 5–15. [Google Scholar] [CrossRef]
- Theakston, R.D.; Warrell, D.A. Antivenoms: A list of hyperimmune sera currently available for the treatment of envenoming by bites and stings. Toxicon 1991, 29, 1419–1470. [Google Scholar] [CrossRef]
- Li, D.; Mattoo, P.; Keller, J.E. New equine antitoxins to botulinum neurotoxins serotypes a and b. Biologicals 2012, 40, 240–246. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Geisbert, J.; Swearengen, J.R.; Jaax, G.P.; Lewis, T.; Huggins, J.W.; Schmidt, J.J.; LeDuc, J.W.; Peters, C.J. Passive immunization of ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Arch. Virol. Suppl. 1996, 11, 135–140. [Google Scholar]
- Schroff, R.W.; Foon, K.A.; Beatty, S.M.; Oldham, R.K.; Morgan, A.C., Jr. Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985, 45, 879–885. [Google Scholar]
- Milenic, D.E.; Detrick, B.; Reynolds, J.C.; Colcher, D. Characterization of primate antibody responses to administered murine monoclonal immunoglobulin. Int. J. Biol. Mark. 1990, 5, 177–187. [Google Scholar]
- Bazin-Redureau, M.I.; Renard, C.B.; Scherrmann, J.M. Pharmacokinetics of heterologous and homologous immunoglobulin g, f(ab’)2 and fab after intravenous administration in the rat. J. Pharm. Pharmacol. 1997, 49, 277–281. [Google Scholar] [CrossRef]
- Olafsen, T.; Wu, A.M. Antibody vectors for imaging. Semin. Nucl. Med. 2010, 40, 167–181. [Google Scholar] [CrossRef]
- Bush, S.P.; Green, S.M.; Moynihan, J.A.; Hayes, W.K.; Cardwell, M.D. Crotalidae polyvalent immune fab (ovine) antivenom is efficacious for envenomations by southern pacific rattlesnakes (crotalus helleri). Ann. Emerg. Med. 2002, 40, 619–624. [Google Scholar] [CrossRef]
- Jones, R.G.; Corteling, R.L.; Bhogal, G.; Landon, J. A novel fab-based antivenom for the treatment of mass bee attacks. Am. J. Trop. Med. Hyg. 1999, 61, 361–366. [Google Scholar]
- Vazquez, H.; Chavez-Haro, A.; Garcia-Ubbelohde, W.; Mancilla-Nava, R.; Paniagua-Solis, J.; Alagon, A.; Sevcik, C. Pharmacokinetics of a f(ab’)2 scorpion antivenom in healthy human volunteers. Toxicon 2005, 46, 797–805. [Google Scholar] [CrossRef]
- Montanaro, L.; Sperti, S.; Stirpe, F. Inhibition by ricin of protein synthesis in vitro. Ribosomes as the target of the toxin. Biochem. J. 1973, 136, 677–683. [Google Scholar]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 s ribosomal rna caused by the toxins. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin poisoning: A comprehensive review. JAMA 2005, 294, 2342–2351. [Google Scholar] [CrossRef]
- Prigent, J.; Panigai, L.; Lamourette, P.; Sauvaire, D.; Devilliers, K.; Plaisance, M.; Volland, H.; Creminon, C.; Simon, S. Neutralising antibodies against ricin toxin. PLoS One 2011, 6, e20166. [Google Scholar] [CrossRef]
- Maddaloni, M.; Cooke, C.; Wilkinson, R.; Stout, A.V.; Eng, L.; Pincus, S.H. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J. Immunol. 2004, 172, 6221–6228. [Google Scholar]
- Yermakova, A.; Mantis, N.J. Protective immunity to ricin toxin conferred by antibodies against the toxin’s binding subunit (rtb). Vaccine 2011, 29, 7925–7935. [Google Scholar] [CrossRef]
- Neal, L.M.; O’Hara, J.; Brey, R.N., 3rd; Mantis, N.J. A monoclonal immunoglobulin g antibody directed against an immunodominant linear epitope on the ricin a chain confers systemic and mucosal immunity to ricin. Infect. Immun. 2010, 78, 552–561. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, L.; Yang, H.; Guo, H.; Fan, K.; Wang, H.; Qian, W.; Zhang, D.; Li, B.; Guo, Y. Identification of a novel functional domain of ricin responsible for its potent toxicity. J. Biol. Chem. 2011, 286, 12166–12171. [Google Scholar]
- Hu, W.G.; Yin, J.; Chau, D.; Hu, C.C.; Lillico, D.; Yu, J.; Negrych, L.M.; Cherwonogrodzky, J.W. Conformation-dependent high-affinity potent ricin-neutralizing monoclonal antibodies. Biomed. Res. Int. 2013, 2013, 471346. [Google Scholar]
- Hu, W.G.; Yin, J.; Chau, D.; Negrych, L.M.; Cherwonogrodzky, J.W. Humanization and characterization of an anti-ricin neutralization monoclonal antibody. PLoS One 2012, 7, e45595. [Google Scholar]
- Lemley, P.V.; Wright, D.C. Mice are actively immunized after passive monoclonal antibody prophylaxis and ricin toxin challenge. Immunology 1992, 76, 511–513. [Google Scholar]
- Chesnut, R.W.; Grey, H.M. Antigen presenting cells and mechanisms of antigen presentation. Crit. Rev. Immunol. 1985, 5, 263–316. [Google Scholar]
- Adamova, E.; Walsh, M.C.; Gosselin, D.R.; Hale, K.; Preissler, M.T.; Graziano, R.F.; Gosselin, E.J. Enhanced antigen-specific antibody and cytokine responses when targeting antigen to human fcgamma receptor type i using an anti-human fcgamma receptor type i-streptavidin fusion protein in an adjuvant-free system. Immunol. Investig. 2005, 34, 417–429. [Google Scholar] [CrossRef]
- Liu, C.; Goldstein, J.; Graziano, R.F.; He, J.; O’Shea, J.K.; Deo, Y.; Guyre, P.M. F(c)gammari-targeted fusion proteins result in efficient presentation by human monocytes of antigenic and antagonist t cell epitopes. J. Clin. Investig. 1996, 98, 2001–2007. [Google Scholar] [CrossRef]
- Guyre, P.M.; Graziano, R.F.; Goldstein, J.; Wallace, P.K.; Morganelli, P.M.; Wardwell, K.; Howell, A.L. Increased potency of fc-receptor-targeted antigens. Cancer Immunol. Immunother. 1997, 45, 146–148. [Google Scholar] [CrossRef]
- Walsh, M.C.; Banas, J.A.; Mudzinski, S.P.; Preissler, M.T.; Graziano, R.F.; Gosselin, E.J. A two-component modular approach for enhancing t-cell activation utilizing a unique anti-fcgammari-streptavidin construct and microspheres coated with biotinylated-antigen. Biomol. Eng. 2003, 20, 21–33. [Google Scholar] [CrossRef]
- Chargelegue, D.; Drake, P.M.; Obregon, P.; Prada, A.; Fairweather, N.; Ma, J.K. Highly immunogenic and protective recombinant vaccine candidate expressed in transgenic plants. Infect. Immun. 2005, 73, 5915–5922. [Google Scholar] [CrossRef]
- Vazquez, H.; Olvera, F.; Alagon, A.; Sevcik, C. Production of anti-horse antibodies induced by igg, f(ab’) and fab applied repeatedly to rabbits. Effect on antivenom pharmacokinetics. Toxicon 2013, 76, 362–369. [Google Scholar] [CrossRef]
- Mayers, C.N.; Veall, S.; Bedford, R.J.; Holley, J.L. Anti-immunoglobulin responses to igg, f(ab’)2, and fab botulinum antitoxins in mice. Immunopharmacol. Immunotoxicol. 2003, 25, 397–408. [Google Scholar] [CrossRef]
- Beyer, N.H.; Kogutowska, E.; Hansen, J.J.; Engelhart Illigen, K.E.; Heegaard, N.H. A mouse model for ricin poisoning and for evaluating protective effects of antiricin antibodies. Clin. Toxicol. (Phila.) 2009, 47, 219–225. [Google Scholar] [CrossRef]
- O'Hara, J.M.; Neal, L.M.; McCarthy, E.A.; Kasten-Jolly, J.A.; Brey, R.N., 3rd; Mantis, N. Folding domains within the ricin toxin a subunit as targets of protective antibodies. Vaccine 2010, 28, 7035–7046. [Google Scholar] [CrossRef]
- Pratt, T.S.; Pincus, S.H.; Hale, M.L.; Moreira, A.L.; Roy, C.J.; Tchou-Wong, K.M. Oropharyngeal aspiration of ricin as a lung challenge model for evaluation of the therapeutic index of antibodies against ricin a-chain for post-exposure treatment. Exp. Lung Res. 2007, 33, 459–481. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, C.C.; Yin, J.; Chau, D.; Cherwonogrodzky, J.W.; Hu, W.-G. Active Immunity Induced by Passive IgG Post-Exposure Protection against Ricin. Toxins 2014, 6, 380-393. https://doi.org/10.3390/toxins6010380
Hu CC, Yin J, Chau D, Cherwonogrodzky JW, Hu W-G. Active Immunity Induced by Passive IgG Post-Exposure Protection against Ricin. Toxins. 2014; 6(1):380-393. https://doi.org/10.3390/toxins6010380
Chicago/Turabian StyleHu, Charles Chen, Junfei Yin, Damon Chau, John W. Cherwonogrodzky, and Wei-Gang Hu. 2014. "Active Immunity Induced by Passive IgG Post-Exposure Protection against Ricin" Toxins 6, no. 1: 380-393. https://doi.org/10.3390/toxins6010380
APA StyleHu, C. C., Yin, J., Chau, D., Cherwonogrodzky, J. W., & Hu, W.-G. (2014). Active Immunity Induced by Passive IgG Post-Exposure Protection against Ricin. Toxins, 6(1), 380-393. https://doi.org/10.3390/toxins6010380



