Silymarin Prevents Restraint Stress-Induced Acute Liver Injury by Ameliorating Oxidative Stress and Reducing Inflammatory Response
Abstract
:1. Introduction
2. Results
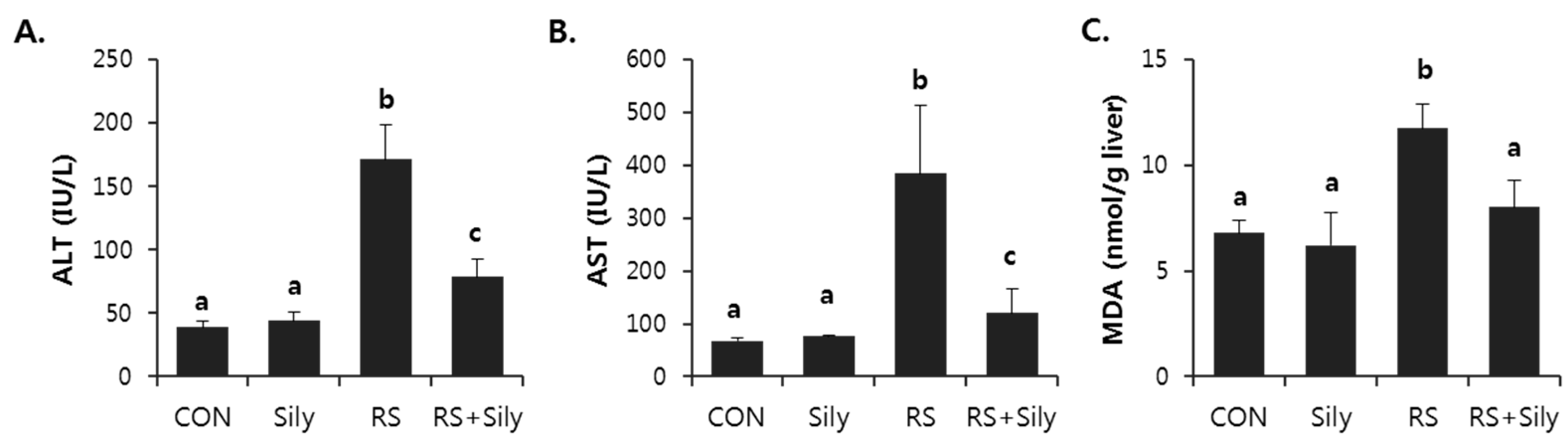
2.1. Effect of Silymarin on Restraint Stress-Induced Acute Liver Injury
2.2. Effect of Silymarin on the Levels of Malondialdehyde (MDA) and 4-Hydroxynonenal (4-HNE) in the Liver of Stressed Mice
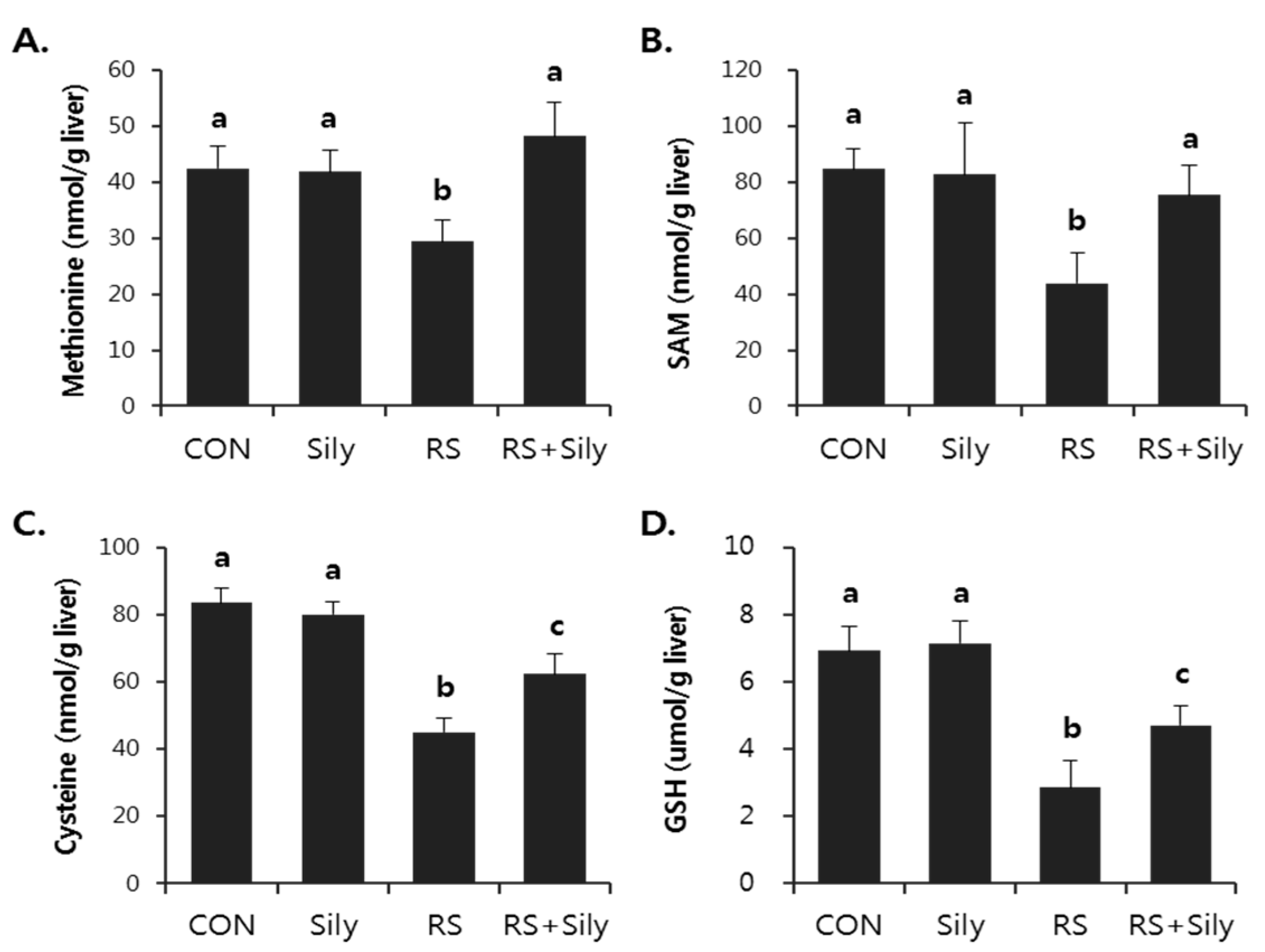
2.3. Effect of Silymarin on Restraint-Induced GSH Depletion
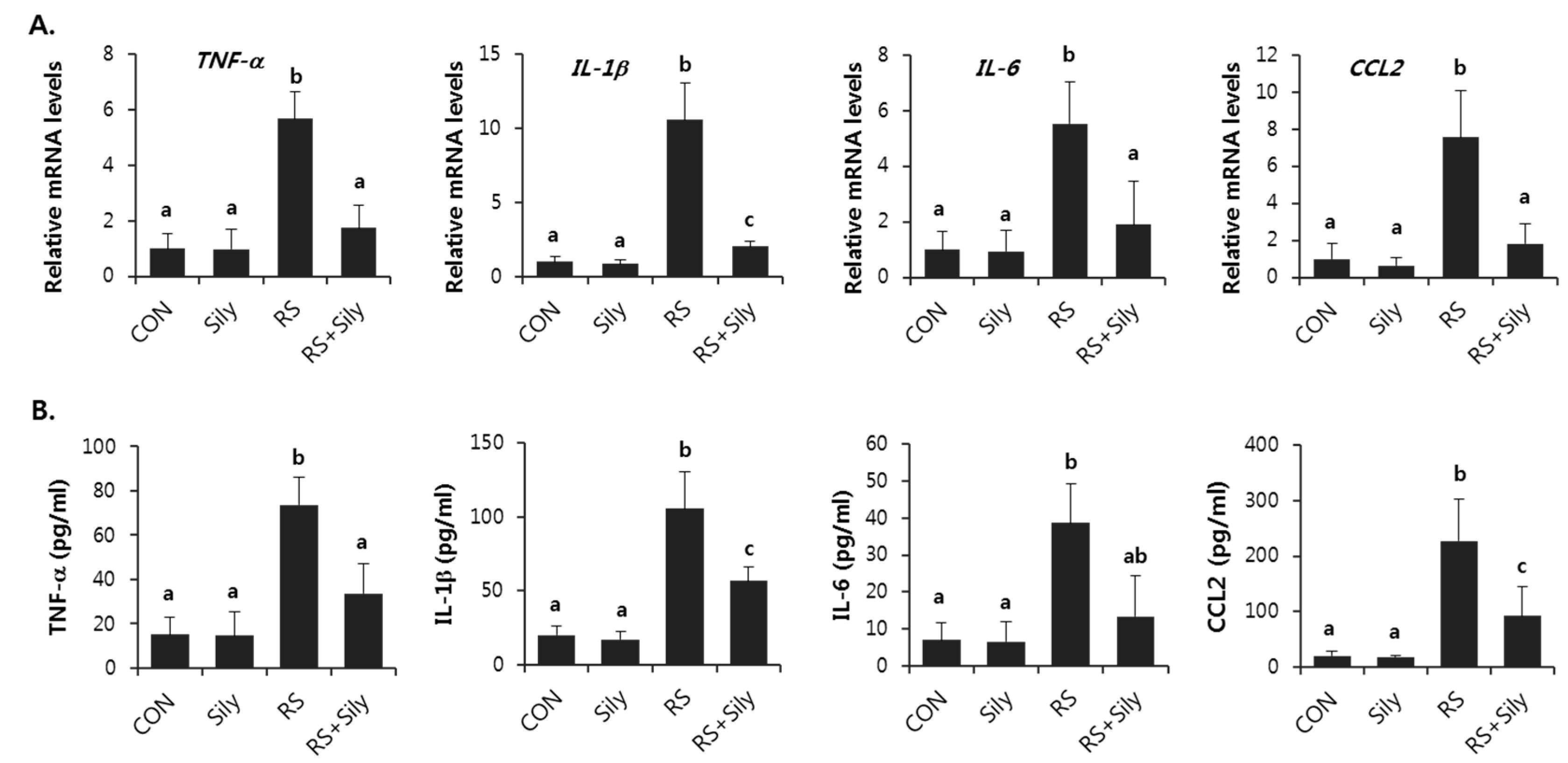
2.4. Effect of Silymarin on the Expression of Inflammatory Mediator Genes in the Liver of Stressed Mice
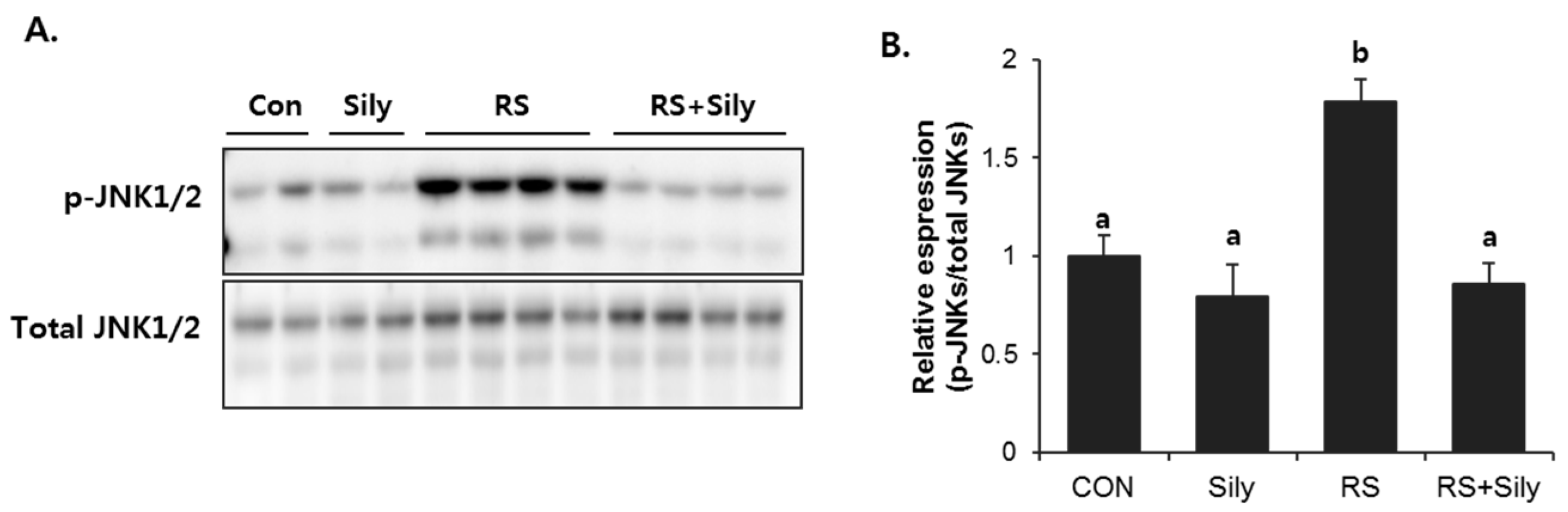
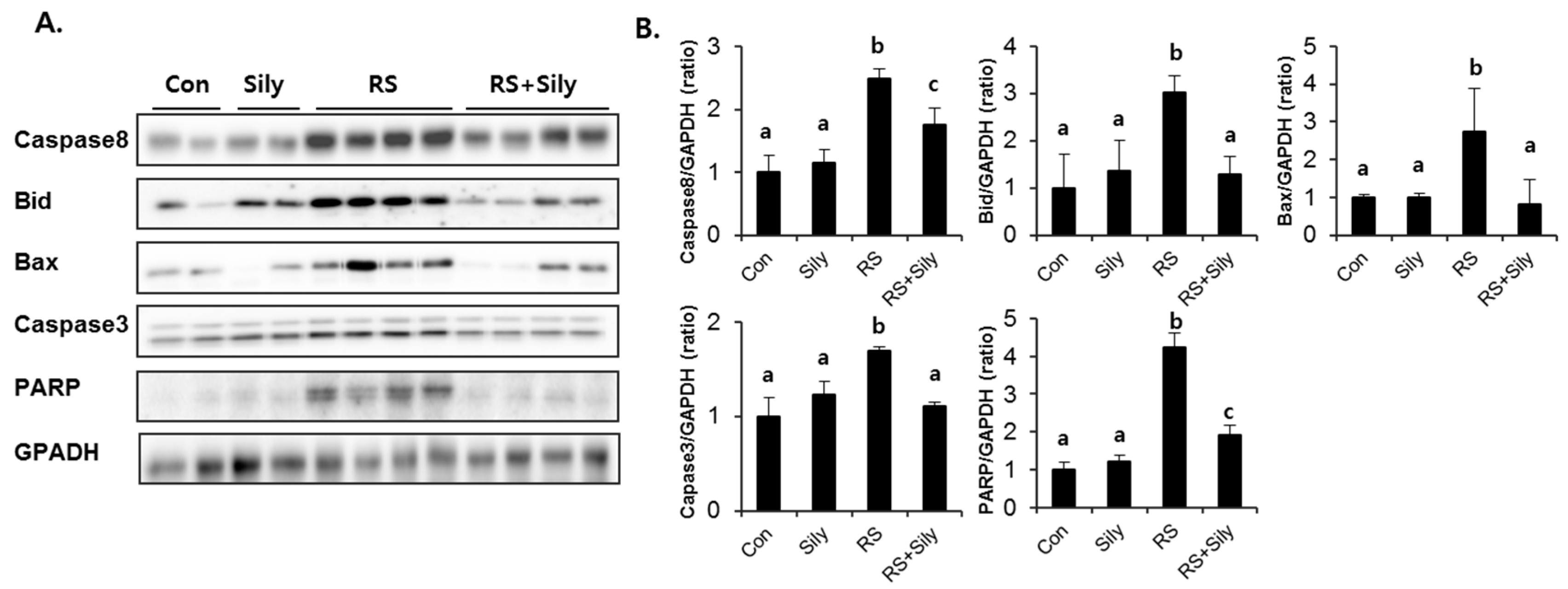
2.5. Effect of Silymarin on JNK Activation and Apoptotic Pathway in the Liver of Stressed Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Determination of Hepatotoxicity and Lipid Peroxidation
4.3. Liver Histopathology
4.4. Determination of Sulfur-Containing Amino Acids
4.5. Measurement of Cytokines in Serum
4.6. RNA Extraction and Real-Time RT-PCR
4.7. Immunoblot Analysis
4.8. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moore, C.J.; Cunningham, S.A. Social position, psychological stress, and obesity: A systematic review. J. Acad. Nutr. Diet. 2012, 112, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, C.; Elsenbruch, S.; Scholle, A.; de Greiff, A.; Schedlowski, M.; Forsting, M.; Gizewski, E.R. Effects of psychological stress on the cerebral processing of visceral stimuli in healthy women. Neurogastroenterol. Motil. 2009, 21, e740–e745. [Google Scholar] [CrossRef]
- Stojanovich, L.; Marisavljevich, D. Stress as a trigger of autoimmune disease. Autoimmun. Rev. 2008, 7, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.J.; Tan, R.R.; He, R.R.; Tang, L.P.; Wang, X.L.; Yao, N.; Duan, W.J.; Hu, Y.A.; Yao, X.S.; Kurihara, H. Sarcandra glabra extract reduces the susceptibility and severity of influenza in restraint-stressed mice. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- He, R.R.; Wang, M.; Wang, C.Z.; Chen, B.T.; Lu, C.N.; Yao, X.S.; Chen, J.X.; Kurihara, H. Protective effect of apple polyphenols against stress-provoked influenza viral infection in restraint mice. J. Agric. Food Chem. 2011, 59, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, B.; He, R.R.; Yang, D.H.; Li, Y.F.; Li, X.D.; Li, W.X.; Abe, K.; Kurihara, H. Carnosine ameliorates stress-induced glucose metabolism disorder in restrained mice. J. Pharmacol. Sci. 2011, 117, 223–229. [Google Scholar] [CrossRef] [PubMed]
- He, R.R.; Yao, N.; Wang, M.; Yang, X.S.; Yau, C.C.; Abe, K.; Yao, X.S.; Kurihara, H. Effects of histamine on lipid metabolic disorder in mice loaded with restraint stress. J. Pharmacol. Sci. 2009, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Yao, X.S.; Yau, C.C.; Tsi, D.; Chia, C.S.; Nagai, H.; Kurihara, H. Protective effects of bilberry (Vaccinium myrtillus L.) extract on restraint stress-induced liver damage in mice. J. Agric. Food Chem. 2008, 56, 7803–7807. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Abe, K.; Tsang, P.; Xu, J.K.; Yao, X.S.; Liu, H.W.; Kurihara, H. Bilberry extract protect restraint stress-induced liver damage through attenuating mitochondrial dysfunction. Fitoterapia 2010, 81, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- He, R.R.; Yao, X.S.; Li, H.Y.; Dai, Y.; Duan, Y.H.; Li, Y.F.; Kurihara, H. The anti-stress effects of sarcandra glabra extract on restraint-evoked immunocompromise. Biol. Pharm. Bull. 2009, 32, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Vere, C.C.; Streba, C.T.; Streba, L.M.; Ionescu, A.G.; Sima, F. Psychosocial stress and liver disease status. World J. Gastroenterol. 2009, 15, 2980–2986. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Kawamura, K.; Takemoto, K. Oxidative damage of nuclear-DNA in liver of rats exposed to psychological stress. Cancer Res. 1993, 53, 4153–4155. [Google Scholar] [PubMed]
- Kim, H.G.; Lee, J.S.; Lee, J.S.; Han, J.M.; Son, C.G. Hepatoprotective and antioxidant effects of Myelophil on restraint stress-induced liver injury in BALB/c mice. J. Ethnopharmacol. 2012, 142, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Rambaldi, A.; Jacobs, B.P.; Iaquinto, G.; Gluud, C. Milk thistle for alcoholic and/or hepatitis b or c liver diseases—A systematic cochrane hepato-biliary group review with meta-analyses of randomized clinical trials. Am. J. Gastroenterol. 2005, 100, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Deaciuc, I.; Song, M.; Lee, D.Y.; Liu, Y.; Ji, X.; McClain, C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol. Clin. Exp. Res. 2006, 30, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, K.; Mohan, C.V.; Gobianand, K.; Karthikeyan, S. Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur. J. Pharmacol. 2007, 560, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Letteron, P.; Labbe, G.; Degott, C.; Berson, A.; Fromenty, B.; Delaforge, M.; Larrey, D.; Pessayre, D. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice. Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochem. Pharmacol. 1990, 39, 2027–2034. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Jung, Y.S.; Kim, S.J.; Kim, Y.S.; Choi, D.W.; Kim, Y.C. Alterations in sulfur amino acid metabolism in mice treated with silymarin: A novel mechanism of its action involved in enhancement of the antioxidant defense in liver. Planta Med. 2013, 79, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 1), 173–179. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. NF-kappab in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, H.; Scales, W.E.; Campbell, D.A., Jr. Tumor necrosis factor alpha alters the cytotoxic effect of hydrogen peroxide in cultured hepatocytes. Biochem. Biophys. Res. Commun. 1997, 230, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Auguet, T.; Vidal, F.; Lopez-Dupla, M.; Broch, M.; Gutierrez, C.; Olona, M.; Oltra, C.; Aguilar, C.; Gonzalez, E.; Quer, J.C.; et al. A study on the TNF-alpha system in caucasian spanish patients with alcoholic liver disease. Drug Alcohol Depend. 2008, 92, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002, 20, S1–S13. [Google Scholar] [PubMed]
- Kumar, R.; Prakash, S.; Chhabra, S.; Singla, V.; Madan, K.; Gupta, S.D.; Panda, S.K.; Khanal, S.; Acharya, S.K. Association of pro-inflammatory cytokines, adipokines & oxidative stress with insulin resistance & non-alcoholic fatty liver disease. Indian J. Med. Res. 2012, 136, 229–236. [Google Scholar] [PubMed]
- Streetz, K.L.; Wustefeld, T.; Klein, C.; Manns, M.P.; Trautwein, C. Mediators of inflammation and acute phase response in the liver. Cell. Mol. Biol. 2001, 47, 661–673. [Google Scholar] [PubMed]
- Nanji, A.A.; Jokelainen, K.; Fotouhinia, M.; Rahemtulla, A.; Thomas, P.; Tipoe, G.L.; Su, G.L.; Dannenberg, A.J. Increased severity of alcoholic liver injury in female rats: Role of oxidative stress, endotoxin, and chemokines. Am. J. Physiol. Gastroint. Liver Physiol. 2001, 281, G1348–G1356. [Google Scholar]
- Zhou, D.; Kusnecov, A.W.; Shurin, M.R.; DePaoli, M.; Rabin, B.S. Exposure to physical and psychological stressors elevates plasma interleukin 6: Relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology 1993, 133, 2523–2530. [Google Scholar] [PubMed]
- Hernandez, J.; Carrasco, J.; Belloso, E.; Giralt, M.; Bluethmann, H.; Kee Lee, D.; Andrews, G.K.; Hidalgo, J. Metallothionein induction by restraint stress: Role of glucocorticoids and IL-6. Cytokine 2000, 12, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Rutledge, B.J.; Gu, L.; Fiorillo, J.; Lukacs, N.W.; Kunkel, S.L.; North, R.; Gerard, C.; Rollins, B.J. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 1998, 187, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Beller, D.I.; Frendl, G.; Graves, D.T. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J. Immunol. 1992, 148, 2423–2428. [Google Scholar] [PubMed]
- Bautista, A.P. Chronic alcohol intoxication primes kupffer cells and endothelial cells for enhanced CC-chemokine production and concomitantly suppresses phagocytosis and chemotaxis. Front. Biosci. 2002, 7, a117–a125. [Google Scholar] [PubMed]
- Mandrekar, P.; Ambade, A.; Lim, A.; Szabo, G.; Catalano, D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: Regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 2011, 54, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N.; Tarugi, P. Improvement in the high-performance liquid chromatography malondialdehyde level determination in normal human plasma. J. Chromatogr. B 1998, 713, 433–437. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Roll, F.J. Glutathione measurement by high-performance liquid chromatography separation and fluorometric detection of the glutathione-orthophthalaldehyde adduct. Anal. Biochem. 1989, 179, 236–241. [Google Scholar] [CrossRef]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, W. High-performance liquid-chromatographic determination of amino-acids in biological samples by precolumn derivatization with O-phthaldialdehyde. J. Liq. Chromatogr. 1987, 10, 941–955. [Google Scholar] [CrossRef]
- She, Q.B.; Nagao, I.; Hayakawa, T.; Tsuge, H. A simple HPLC method for the determination of S-adenosylmethionine and S-adenosylhomocysteine in rat-tissues—The effect of vitamin-B-6 deficiency on these concentrations in rat-liver. Biochem. Biophys. Res. Commun. 1994, 205, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Silymarin is available from Sigma Aldrich (Cat. No. S0292).

| Genes | Primer Sequences | |
|---|---|---|
| TNF-α | F: GGCCTCTCTACCTTGTTGCC | R: CAGCCTGGTCACCAAATCAG |
| IL-1β | F: TTCACCATGGAATCCGTGTC | R: GTCTTGGCCGAGGACTAAGG |
| IL-6 | F: TTGCCTTCTTGGGACTGATG | R: CCACGATTTCCCAGAGAACA |
| CCl2 | F: CCAGCAAGATGATCCCAATG | R: CTTCTTGGGGTCAGCACAGA |
| 18S | F: CAGCCACCCGAGATTGAGCA | R: TAGTAGCGACGGGCGGTGTG |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Oh, D.-S.; Oh, J.Y.; Son, T.G.; Yuk, D.Y.; Jung, Y.-S. Silymarin Prevents Restraint Stress-Induced Acute Liver Injury by Ameliorating Oxidative Stress and Reducing Inflammatory Response. Molecules 2016, 21, 443. https://doi.org/10.3390/molecules21040443
Kim SH, Oh D-S, Oh JY, Son TG, Yuk DY, Jung Y-S. Silymarin Prevents Restraint Stress-Induced Acute Liver Injury by Ameliorating Oxidative Stress and Reducing Inflammatory Response. Molecules. 2016; 21(4):443. https://doi.org/10.3390/molecules21040443
Chicago/Turabian StyleKim, Sou Hyun, Dal-Seok Oh, Ji Youn Oh, Tae Gen Son, Dong Yeon Yuk, and Young-Suk Jung. 2016. "Silymarin Prevents Restraint Stress-Induced Acute Liver Injury by Ameliorating Oxidative Stress and Reducing Inflammatory Response" Molecules 21, no. 4: 443. https://doi.org/10.3390/molecules21040443





