Secreted Non-Coding RNAs as Signaling Molecules Driving Cell-to-Cell Communication in Cancer
A topical collection in Non-Coding RNA (ISSN 2311-553X).
Viewed by 10604Editor
Interests: microRNAs; lncRNAs; circRNA; tumor angiogenesis; tumor-associated macrophages; cancer biology; p53
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
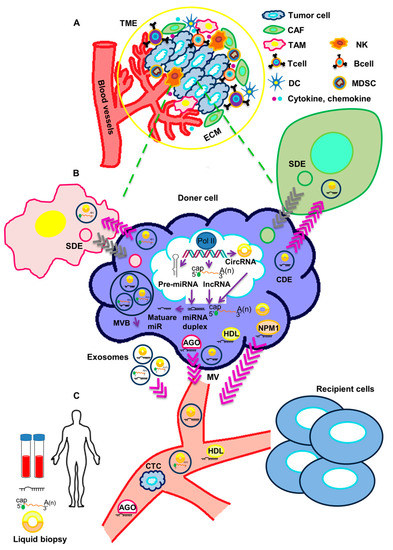
It is becoming increasingly clear that cancer cells actively communicate with a variety of cell types through the release of soluble mediators. This is crucial to the acquisition of diverse cancer-associated features, such as the ability to inhibit the immune recognition of malignant cells, the remodeling of the extracellular matrix (ECM), the establishment of an inflammatory state, the alteration of vascular permeability, and the preparation of the metastatic niche.
For decades, our studies have been focused on protein mediators, such as cytokines and chemokines, which strongly contribute to immune escape but also to neoangiogenesis in cancer.
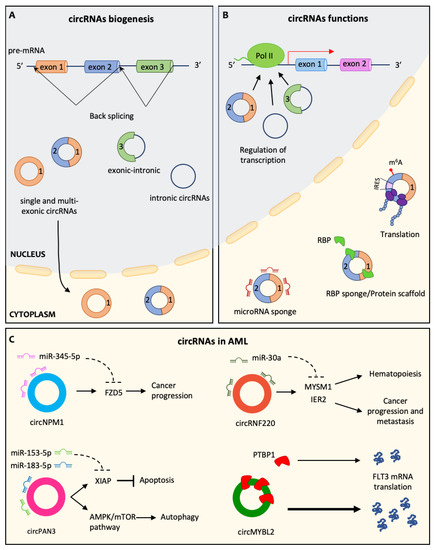
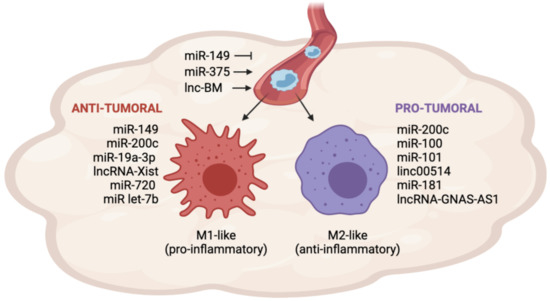
More recently, cancer cells have been shown to have the ability to release non-coding RNAs (ncRNAs), such as microRNAs, long non-coding RNAs, and circular RNAs. ncRNAs are secreted in vesicles or associated with cargo proteins and might be involved in autocrine as well as in paracrine signaling networks, finally leading to the alteration of target cells' behavior in the protumorigenic sense. The targeting of ncRNAs, which mediate the cross-talk between cancer cells and other cells in the tumor microenvironement, is emerging as a promising approach to enhancing traditional anticancer treatments.
The purpose of this Topical Collection is to present a collection of articles (both original research articles and reviews) from experts in non-coding RNA research that highlight the important function of ncRNAs in cell-to-cell communication.
Dr. Giulia FontemaggiCollection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Non-Coding RNA is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1800 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- microRNA, circRNA, and lncRNA in the tumor microenvironement
- immune escape
- liquid biopsy
- extracellular matrix remodeling
- chemoresistance
- radioresistance