Marine Drugs in the Management of Metabolic Diseases
A topical collection in Marine Drugs (ISSN 1660-3397).
Viewed by 120303Editor
Interests: bioactivity screening; natural products; elucidation of molecular mechanism; obesity and related diseases; cyanobacteria bioactive compounds
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Metabolic diseases consist of complex heterogeneous disorders caused by the breakdown of body homeostasis, which greatly affect individuals’ quality of life. Mainly due to significant changes in human lifestyles, such as sedentarism, excess calories, and exponential growth of emotional stress, metabolic diseases have been the current target of public health and scientific concern.
Marine organisms are rich sources of bioactive compounds with diverse molecular structures and biological activities, which place them at the forefront as the most appealing sources of drug leads in the 21st century. In addition to constituting privileged targets of chemical and molecular studies, marine drugs have a privileged role in pharmaceutical bioprospecting, representing a new hope for the management of metabolic diseases.
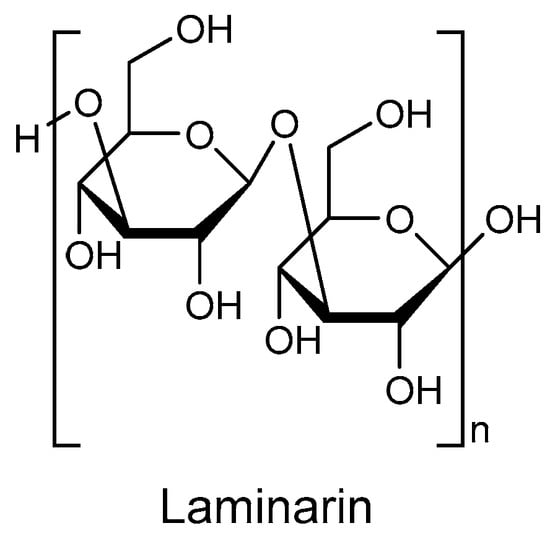
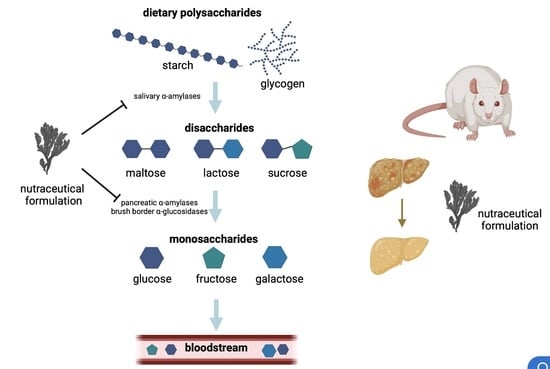
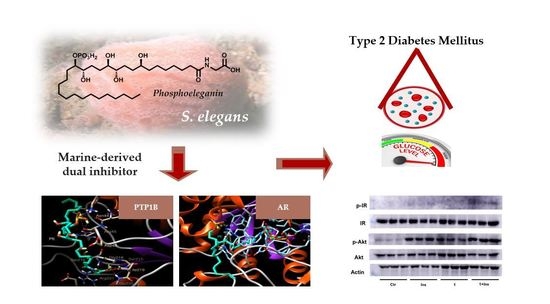
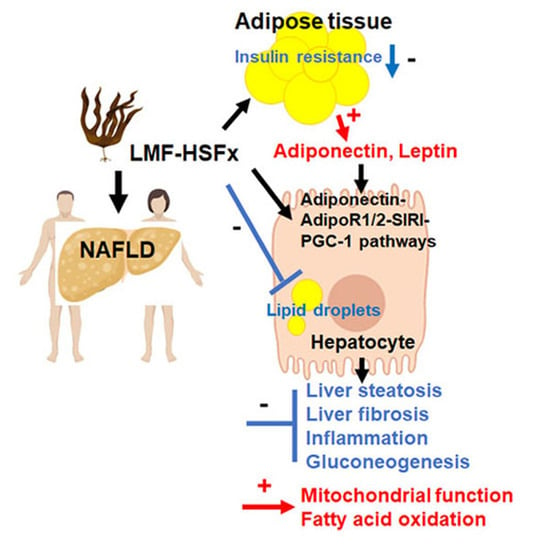
In this Topical Collection, we are seeking original and high-quality research articles, as well as reviews, focused on marine drugs with potential for the pharmacological management of metabolic diseases, with emphasis on obesity, diabetes, hepatic steatosis, dyslipidemia, hypertension, and others. We intend to highlight surveys concerning novel compounds from marine sources, with relevant prospects on metabolic diseases related processes or concerning known compounds with advances on the deciphering of the molecular mode of action.
Dr. Graciliana Lopes
Former Collection Editor
[email protected]
Affiliation: CIIMAR/CIMAR, Interdisciplinary Centre of Marine and Environmental Research, Novo Edifício do Terminal de Cruzeiros do Porto de Leixões, Avenida General Norton de Matos, S/N, 4450-208 Matosinhos, Portugal
Interests: marine drugs; drug discovery; Diabetes mellitus; oxidative stress; seaweed bioactive compounds; phlorotannins
Dr. Ralph Urbatzka
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Marine Drugs is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- metabolic diseases (obesity, diabetes, steatosis, dyslipidemia, hypertension, etc.)
- mechanism of action
- compounds isolation and molecular modulation
- structure-activity relationship