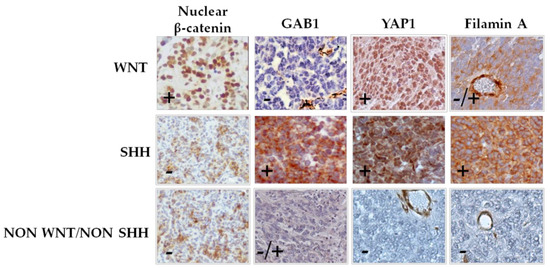
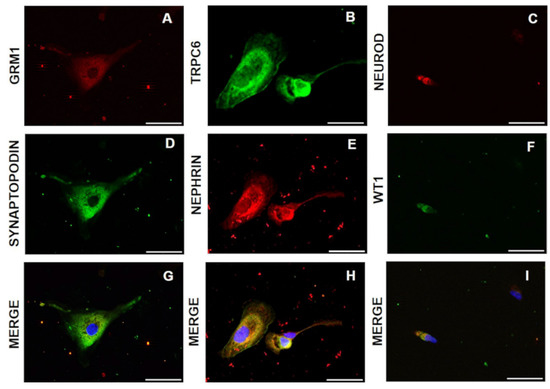
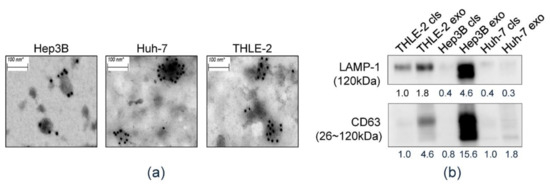
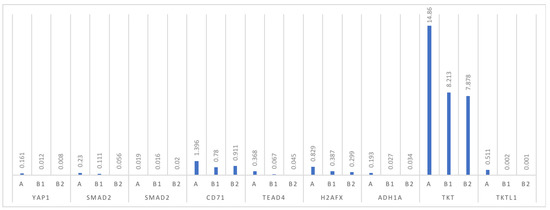
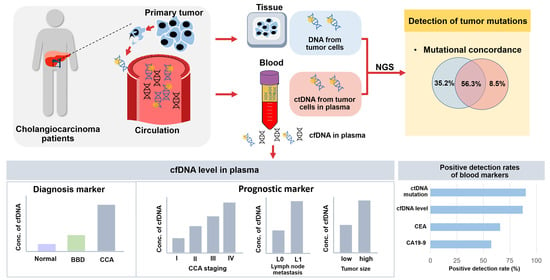
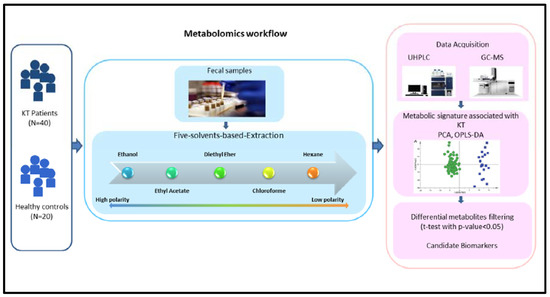
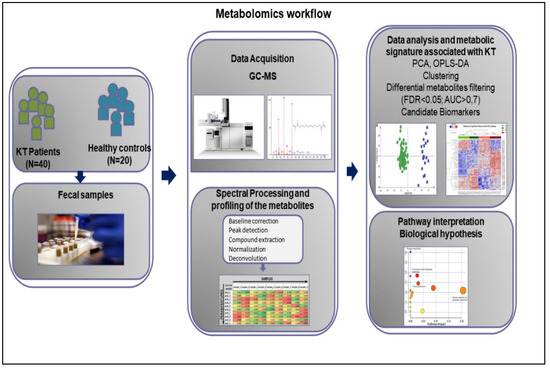
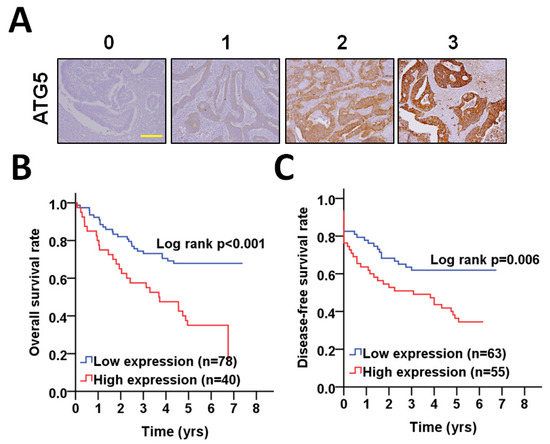
Biomarkers in Medicine
A topical collection in Diagnostics (ISSN 2075-4418). This collection belongs to the section "Pathology and Molecular Diagnostics".
Viewed by 175394Editors
Interests: biophysics; amyloid formation; amyloid cytotoxicity; protein misfolding; amyloid-neuroinflammatory cascade; amyloid diagnostics and prognostics; neurodegenerative diseases
Special Issues, Collections and Topics in MDPI journals
Interests: biomarkers; cancer; metastasis; bone healing; angiogenesis; inflammation; cell–cell interactions; proteinases; growth factors; extracellular vesicles; diagnosis; prognosis; imaging; animal models
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The Topical Collection, "Biomarkers", will be focused on the diagnostics of a plethora of pathologies using biomarkers in the blood, cerebrospinal fluid (CSF), and other body fluids. These diseases include widely-spread ailments, such as cancer, diabetes, neurodegenerative Alzheimer’s, Parkinson’s, as well as other pathological conditions. Due to the growing elderly population, these diseases are on the rise in modern society and combatting them is a global issue. Early, or even pre-clinical, diagnostics of pathological conditions at the stage when the clinical symptoms are not yet obvious are of significant therapeutic value, and would enable administration of protective or preventive treatments. This may lead to a significant reduction in the social and healthcare costs of these diseases, as early diagnostics will culminate in more efficient treatment.
The molecular constitution of blood and other body fluids has been shown to be highly representative of the physiological state of an individual. Body-fluid specimen analyses (liquid biopsies) are minimally invasive and a relatively cheap means of early disease detection and convenient monitoring of disease response to therapeutic intervention. These approaches are tightly aligned with the concept of personalized healthcare. Over the last few years, a range of new biomarkers, associated with disease pathology, were discovered, and an arsenal of novel robust diagnostic assays for the (presymptomatic) detection of pathological conditions was established. Biodiagnostics is a rapidly expanding field, yet many questions and problems need to be solved. Some soluble candidate biomarkers failed to demonstrate clinical utility, which may be caused by insufficient association with the disease, or because their validation has been limited to a subset of patients, or by technical limitations in the detection methods.
The present Topical Collection aims at bringing primary research and review articles together to summarize state-of-the-art problems, solutions, and future directions in the biomarker and biodiagnostics field, based on the analysis of various body fluids. Your contribution is very welcome!
Prof. Dr. Ludmilla Morozova-Roche
Dr. Cornelis F.M. Sier
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Diagnostics is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- ascites
- biomarkers
- biosensor assay
- diagnostics
- blood analysis
- cancer
- cerebrospinal fluid
- circulating tumor cell
- DNA, miRNA
- diabetes
- disease monitoring
- exosome
- liquid biopsy
- microvesicle
- neoplasia
- neurodegenerative disease
- metastasis
- plasma
- point of care device
- presymptomatic detection
- serum
- sputum
- tumor marker
- urine