A New {Dy5} Single-Molecule Magnet Bearing the Schiff Base Ligand N-Naphthalidene-2-amino-5-chlorophenol
Abstract
:1. Introduction
2. Results and Discussion
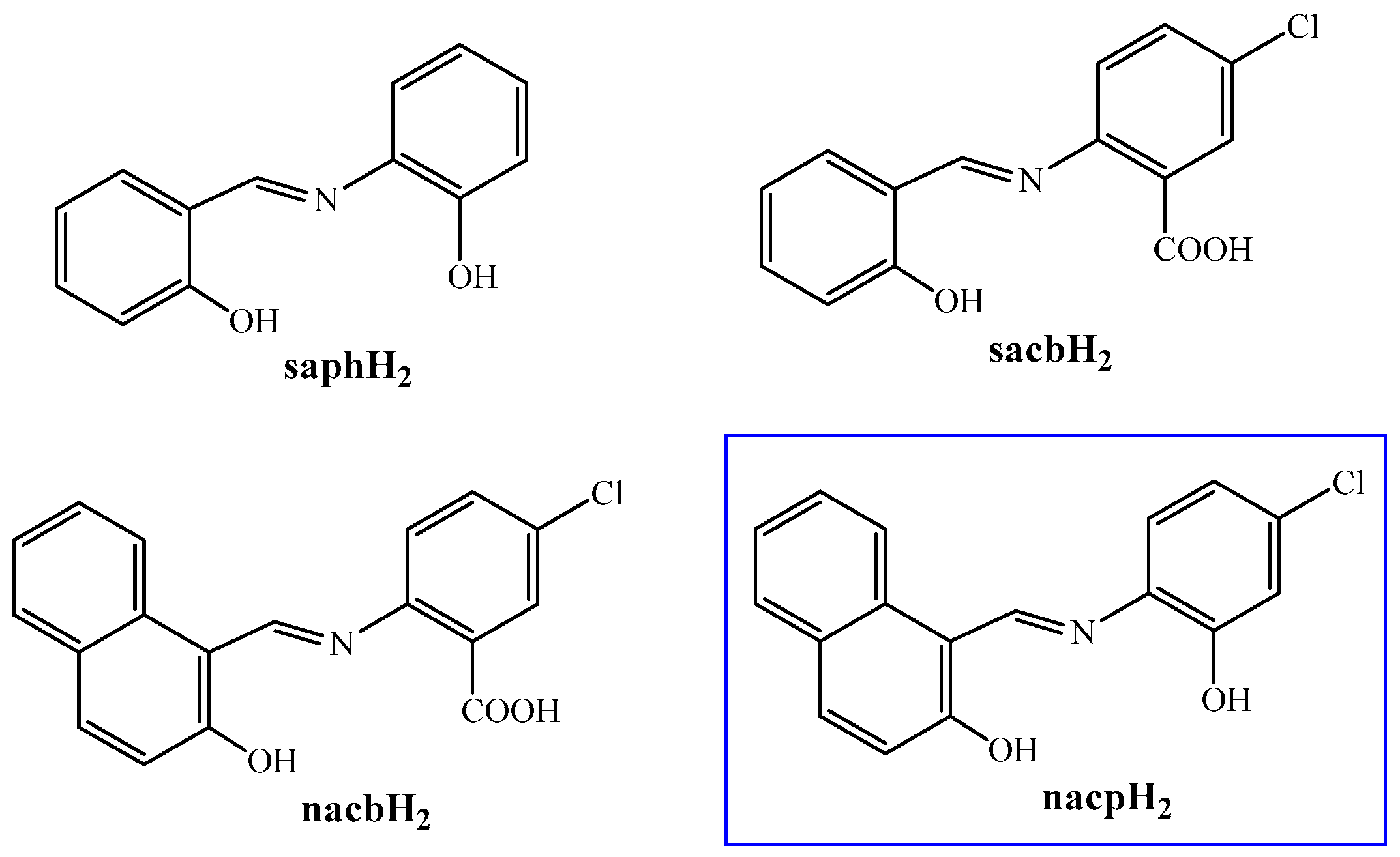
2.1. Synthetic Comments
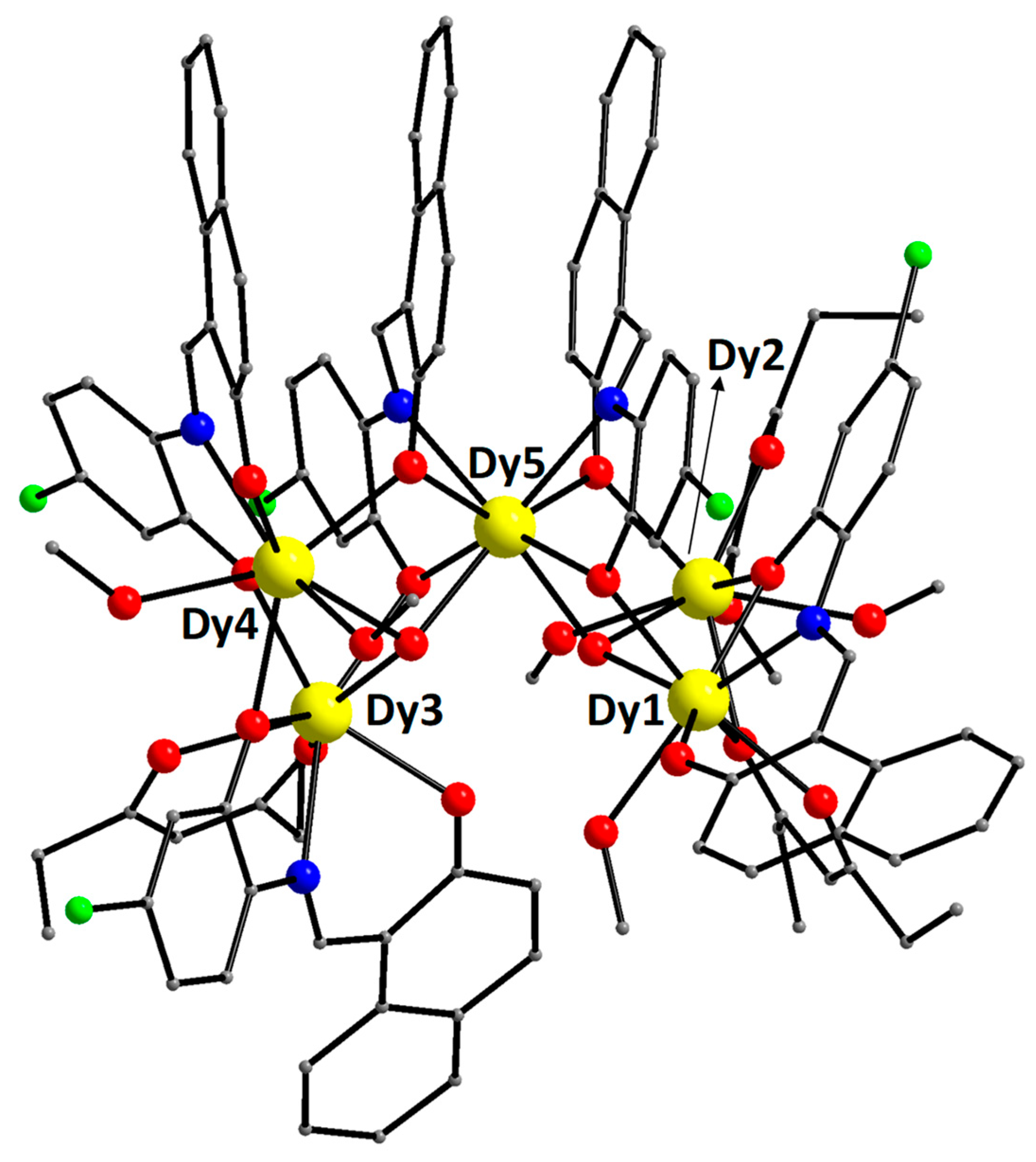
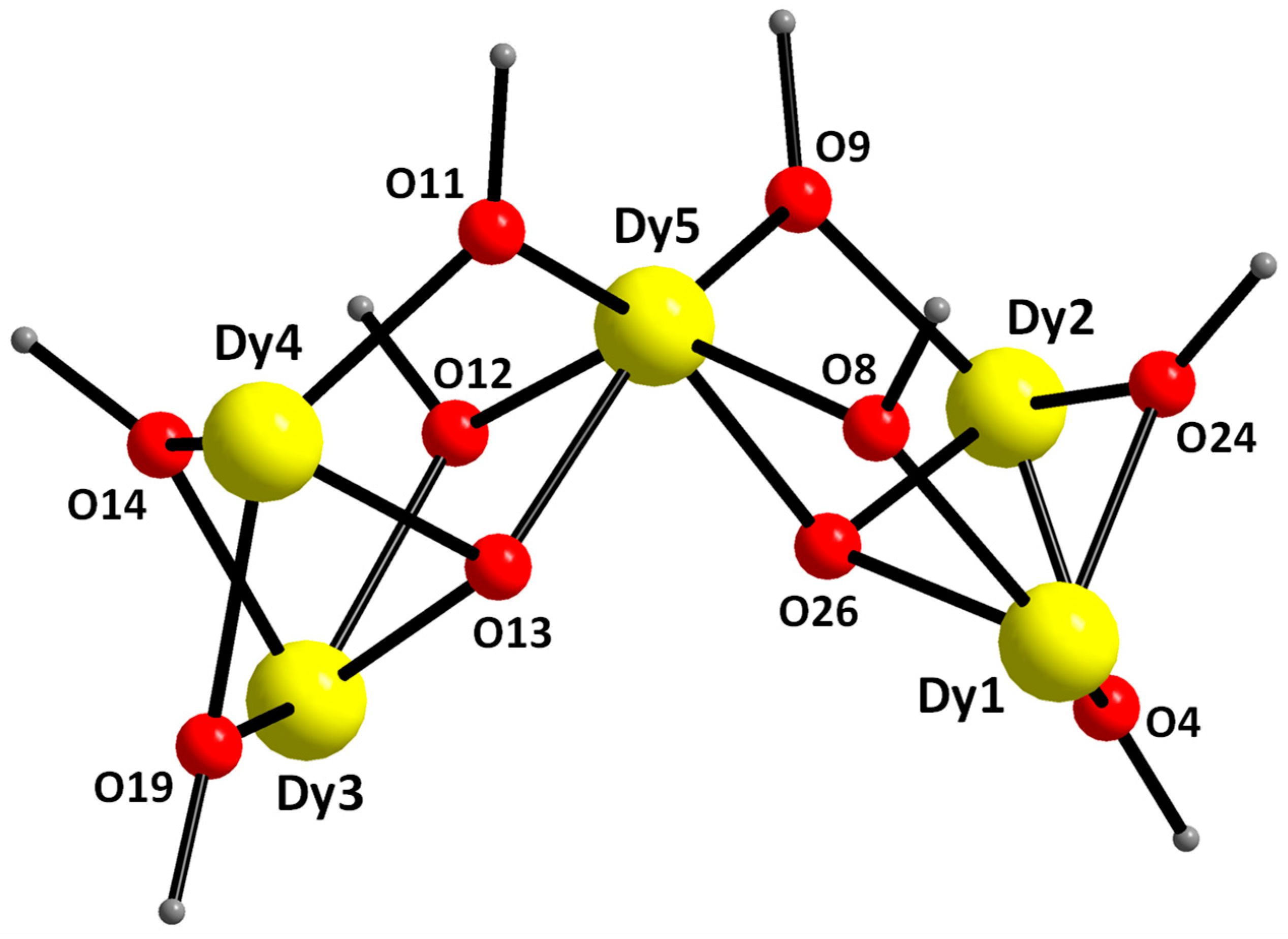
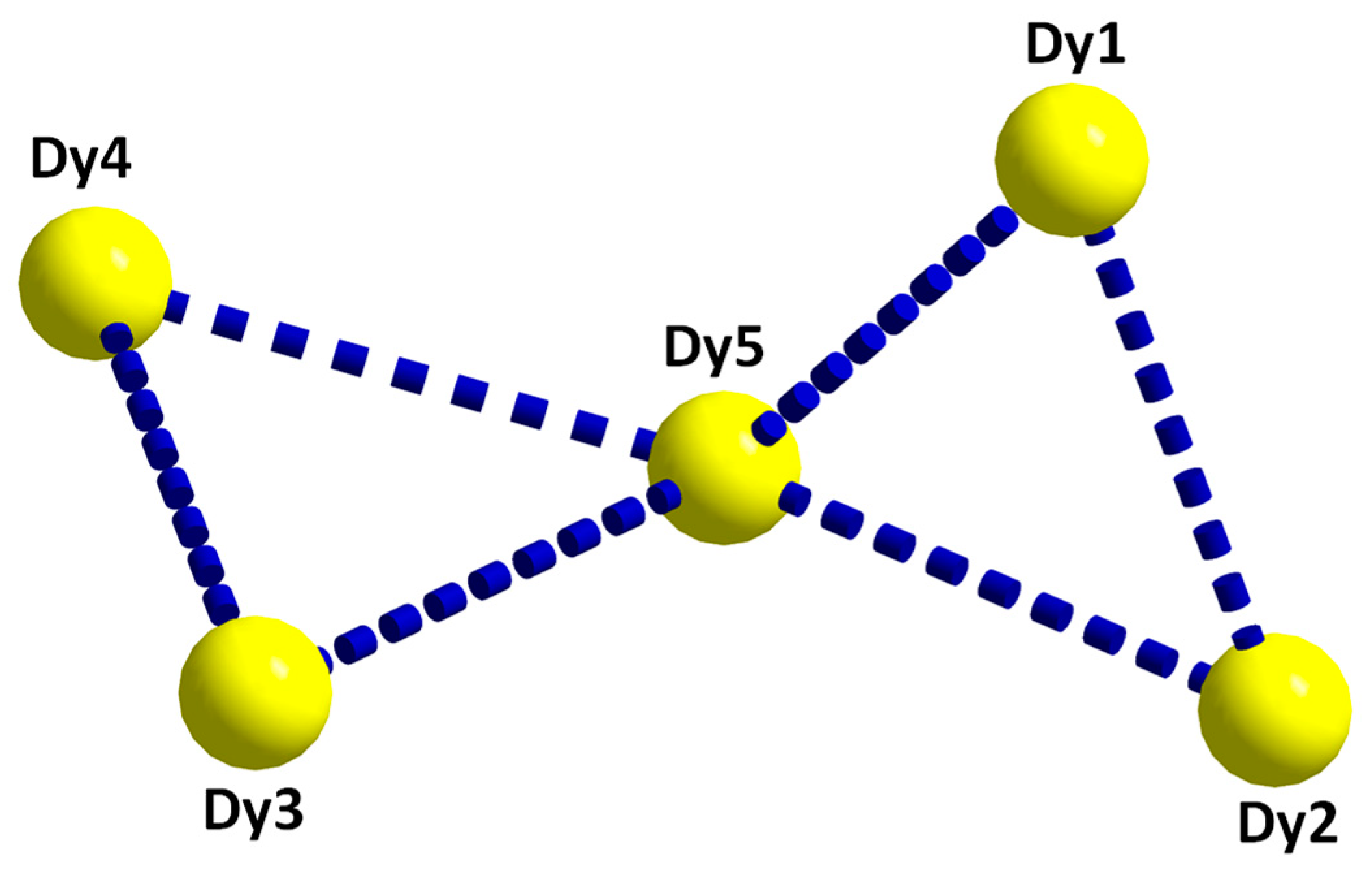
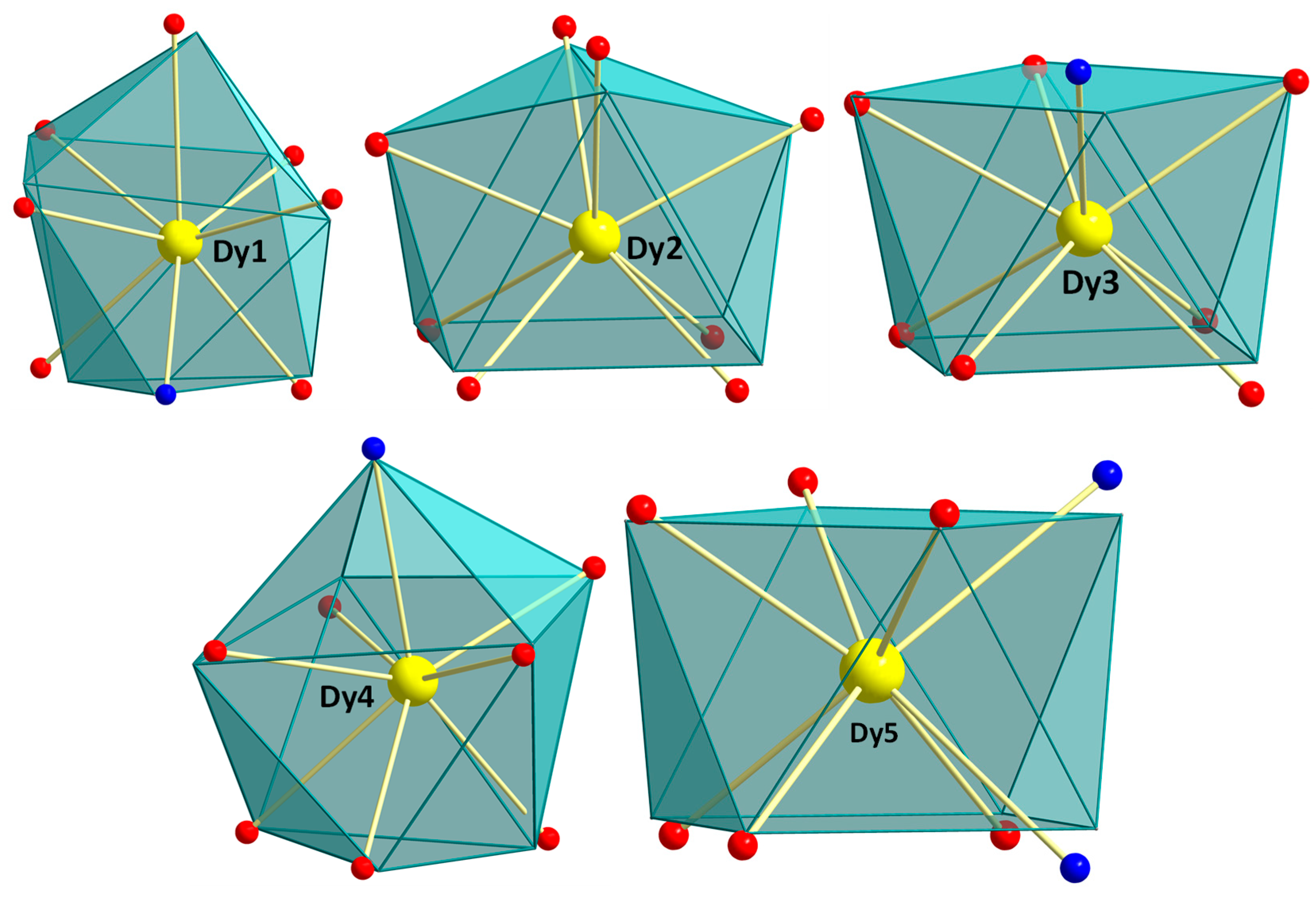
2.2. Description of Structure
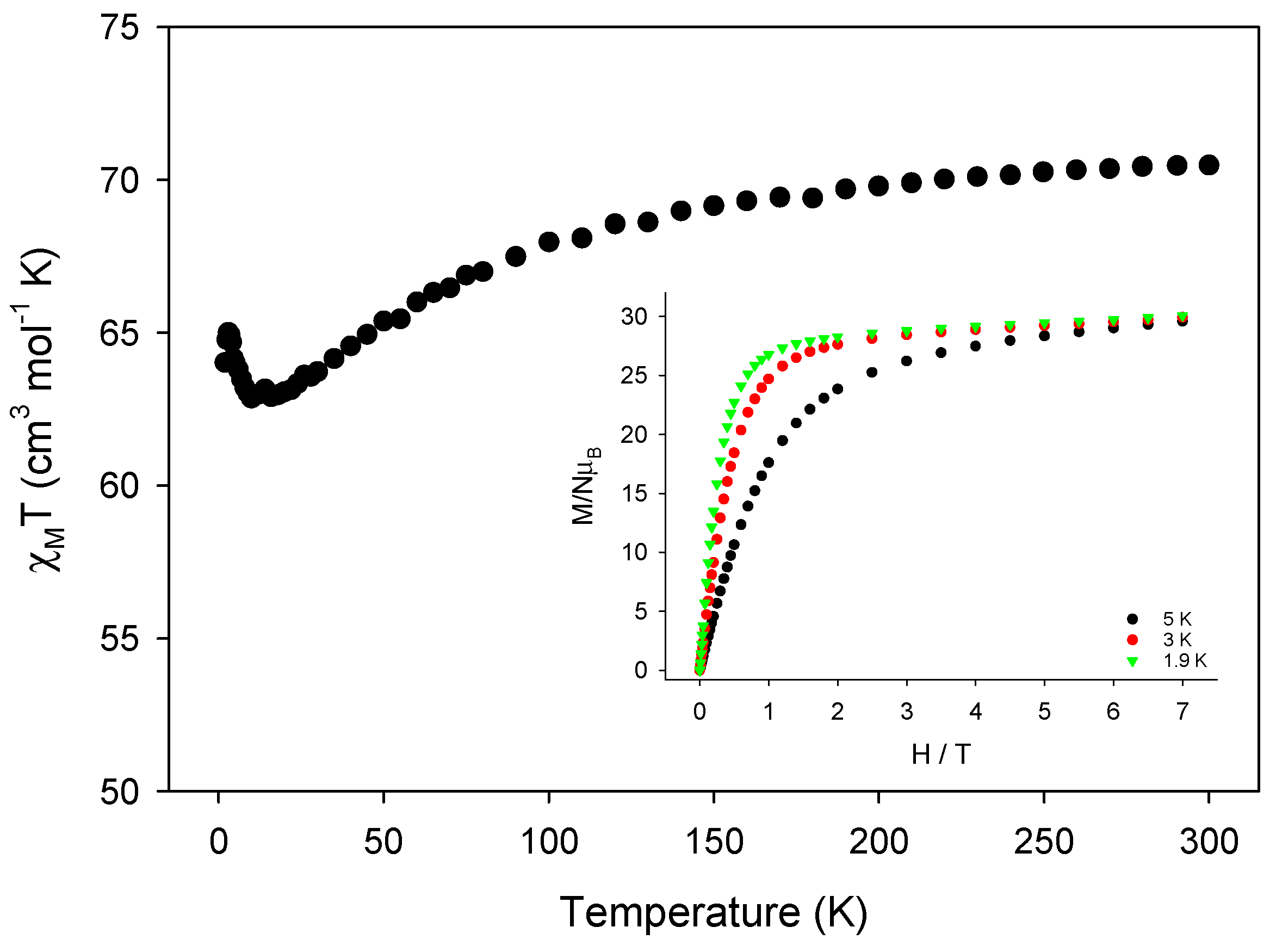
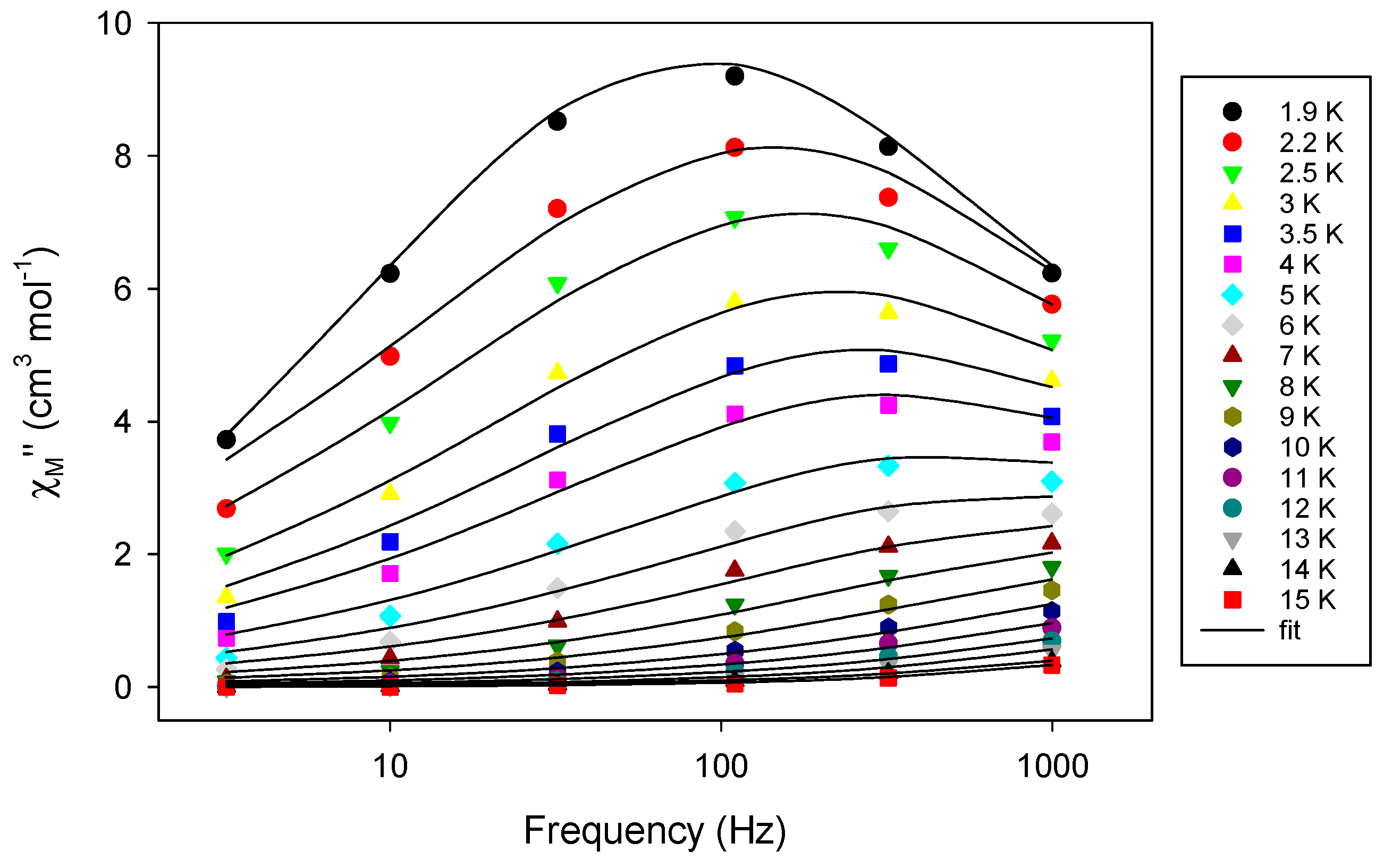
2.3. Solid-State Magnetic Susceptibility Studies
3. Experimental Section
3.1. Materials, Physical and Spectroscopic Measurements
3.2. Synthesis of nacpH2
3.3. Synthesis of [Dy5(OH)2(hpd)3(nacp)5(MeOH)5] (1)
3.4. Single-Crystal X-ray Crystallography
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peng, J.-B.; Kong, X.-J.; Zhang, Q.-C.; Orendac, M.; Prokleska, J.; Ren, Y.-P.; Long, L.-S.; Zheng, Z.; Zheng, L.-S. Beauty, Symmetry, and Magnetocaloric Effect—Four-Shell Keplerates with 104 Lanthanide Atoms. J. Am. Chem. Soc. 2014, 136, 17938–17941. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G.; Eliseeva, S.V. Basics of Lanthanide Photophysics. In Lanthanide Luminescence; Springer Series on Fluorescence; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–45. [Google Scholar]
- Tang, J.; Zhang, P. Lanthanide Single Molecule Magnets; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Layfield, R.A.; Murugesu, M. Lanthanides and Actinides in Molecular Magnetism; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Bogani, L.; Wernsdorfer, W. Molecular Spintronics Using Single-Molecule Magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sessoli, R.; Powell, A.K. Strategies towards Single Molecule Magnets Based on Lanthanide Ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Y.-N.; Tang, J. Recent Advances in Dysprosium-based Single Molecule Magnets: Structural Overview and Synthetic Strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting Single-Ion Anisotropy in the Design of f-Element Single-Molecule Magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Mazarakioti, E.C.; Cunha-Silva, L.; Bekiari, V.; Escuer, A.; Stamatatos, T.C. New Structural Topologies in 4f-Metal Cluster Chemistry from Vertex-sharing Butterfly Units: {LnIII7} Complexes Exhibiting Slow Magnetization Relaxation and Ligand-centred Emissions. RSC Adv. 2015, 5, 92534–92538. [Google Scholar] [CrossRef]
- Mazarakioti, E.C.; Regier, J.; Cunha-Silva, L.; Wernsdorfer, W.; Pilkington, M.; Tang, J.; Stamatatos, T.C. Large Energy Barrier and Magnetization Hysteresis at 5 K for a Symmetric {Dy2} Complex with Spherical Tricapped Trigonal Prismatic DyIII Ions. Inorg. Chem. 2017, 56, 3568–3578. [Google Scholar] [CrossRef] [PubMed]
- Perlepe, P.S.; Cunha-Silva, L.; Gagnon, K.J.; Teat, S.J.; Lampropoulos, C.; Escuer, A.; Stamatatos, T.C. “Ligands-with-Benefits”: Naphthalene-Substituted Schiff Bases Yielding New NiII Metal Clusters with Ferromagnetic and Emissive Properties and Undergoing Exciting Transformations. Inorg. Chem. 2016, 55, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Murugesu, M. Lessons Learned from Dinuclear Lanthanide Nano-magnets. Chem. Soc. Rev. 2013, 42, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Brunet, G.; Vieru, V.; Korobkov, I.; Chibotaru, L.F.; Murugesu, M. Significant Enhancement of Energy Barriers in Dinuclear Dysprosium Single-Molecule Magnets through Electron-Withdrawing Effects. J. Am. Chem. Soc. 2013, 135, 13242–13245. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhao, L.; Lin, H.; Tang, J.; Li, G. Butterfly-Shaped Pentanuclear Dysprosium Single-Molecule Magnets. Chem. Eur. J. 2013, 19, 13235–13241. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Das, S.; van Leusen, J.; Kögerler, P.; Chandrasekhar, V. Pentanuclear [2.2] Spirocyclic Lanthanide(III) Complexes: Slow Magnetic Relaxation of the DyIII Analogue. Dalton Trans. 2015, 44, 19282–19293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, M.; Mondal, A.; Mereacre, V.; Kumar Jana, S.; Powell, A.K.; Roesky, P.W. Tetranuclear and Pentanuclear Compounds of the Rare-Earth Metals: Synthesis and Magnetism. Inorg. Chem. 2015, 54, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, A.; Kundu, S.; Biswas, S.; Suriya Narayanan, R.; Titos-Padilla, S.; Lorusso, G.; Evangelisti, M.; Colacio, E.; Chandrasekhar, V. Decanuclear Ln10 Wheels and Vertex-Shared Spirocyclic Ln5 Cores: Synthesis, Structure, SMM Behavior, and MCE Properties. Chem. Eur. J. 2015, 21, 16955–16967. [Google Scholar] [CrossRef] [PubMed]
- Llunell, M.; Casanova, D.; Girera, J.; Alemany, P.; Alvarez, S. SHAPE, version 2.0; SHAPE: Barcelona, Spain, 2010. [Google Scholar]
- Zabrodsky, H.; Peleg, S.; Avnir, D. Continuous Symmetry Measures. 2. Symmetry Groups and the Tetrahedron. J. Am. Chem. Soc. 1993, 115, 8278–8289. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Demir, S.; Zadrozny, J.M.; Nippe, M.; Long, J.R. Exchange Coupling and Magnetic Blocking in Bipyrimidyl Radical-Bridged Dilanthanide Complexes. J. Am. Chem. Soc. 2012, 134, 18546–18549. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Liddle, S.T.; Van Slageren, J. Improving f-Element Single Molecule Magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Lin, P.-H.; Long, J.; Korobkov, I.; Wernsdorfer, W.; Murugesu, M. The Use of Magnetic Dilution to Elucidate the Slow Magnetic Relaxation Effects of a Dy2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 8830–8833. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Guo, Y.; Tang, J. Relaxation Dynamics of Dysprosium(III) Single Molecule Magnets. Dalton Trans. 2011, 40, 9953–9963. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.F.; Wagner, W.F.; Sands, D.E. Anhydrous and Hydrated Rare Earth Acetylacetonates and Their Infrared Spectra. Inorg. Chem. 1968, 7, 2495–2500. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bradenburg, K.; Putz, H. DIAMOND, Release 3.1f, Crystal Impact GbR; DIAMOND: Bonn, Germany, 2008. [Google Scholar]
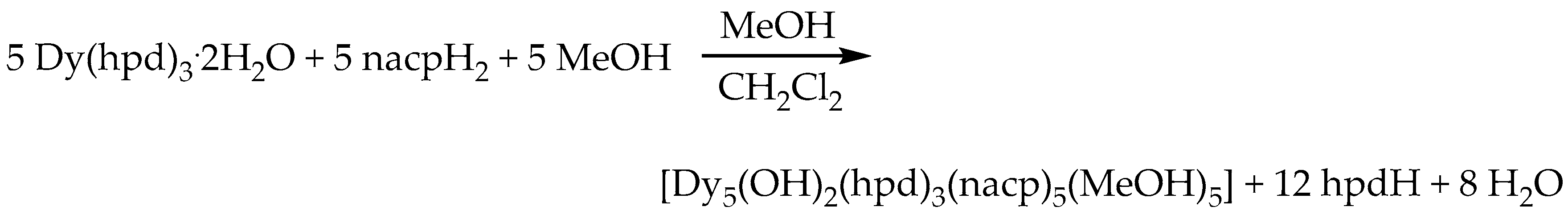




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandropoulos, D.I.; Alaimo, A.A.; Sun, D.; Stamatatos, T.C. A New {Dy5} Single-Molecule Magnet Bearing the Schiff Base Ligand N-Naphthalidene-2-amino-5-chlorophenol. Magnetochemistry 2018, 4, 48. https://doi.org/10.3390/magnetochemistry4040048
Alexandropoulos DI, Alaimo AA, Sun D, Stamatatos TC. A New {Dy5} Single-Molecule Magnet Bearing the Schiff Base Ligand N-Naphthalidene-2-amino-5-chlorophenol. Magnetochemistry. 2018; 4(4):48. https://doi.org/10.3390/magnetochemistry4040048
Chicago/Turabian StyleAlexandropoulos, Dimitris I., Alysha A. Alaimo, Di Sun, and Theocharis C. Stamatatos. 2018. "A New {Dy5} Single-Molecule Magnet Bearing the Schiff Base Ligand N-Naphthalidene-2-amino-5-chlorophenol" Magnetochemistry 4, no. 4: 48. https://doi.org/10.3390/magnetochemistry4040048




