Self-Assembly Properties of Amphiphilic Iron(III) Spin Crossover Complexes in Water and at the Air–Water Interface
Abstract
:1. Introduction
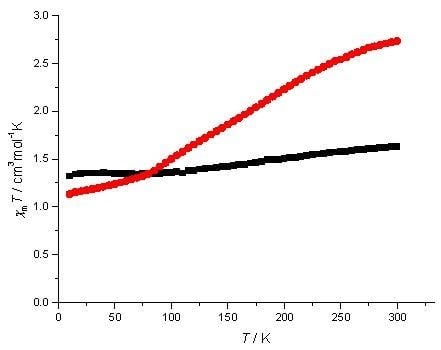
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weller, D.; Moser, A. Thermal effect limits in ultrahigh-density magnetic recording. IEEE Trans. Magn. 1999, 35, 4423–4439. [Google Scholar] [CrossRef]
- Skumryev, V.; Stoyanov, S.; Zhang, Y.; Hadjipanayis, G.; Givord, D.; Nogués, J. Beating the superparamagnetic limit with exchange bias. Nature 2003, 423, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Leoni, T.; Guillermet, O.; Walch, H.; Langlais, V.; Scheuermann, A.; Bonvoisin, J.; Gauthier, S. Controlling the Charge State of a Single Redox Molecular Switch. Phys. Rev. Lett. 2011, 106, 216103. [Google Scholar] [CrossRef] [PubMed]
- Swart, I.; Sonnleitner, T.; Repp, J. Charge State Control of Molecules Reveals Modification of the Tunneling Barrier with Intramolecular Contrast. Nano Lett. 2011, 11, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Repp, J.; Meyer, G.; Olsson, F.E.; Persson, M. Controlling the Charge State of Individual Gold Adatoms. Science 2004, 305, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Létard, J.-F.; Guionneau, P.; Goux-Capes, L. Towards Spin Crossover Applications. Top. Curr. Chem. 2004, 235, 221–249. [Google Scholar] [CrossRef]
- Brusso, J.L.; Clements, O.P.; Haddon, R.C.; Itkis, M.E.; Leitch, A.A.; Oakley, R.T.; Reed, R.W.; Richardson, J.F. Bistabilities in 1,3,2-Dithiazolyl Radicals. J. Am. Chem. Soc. 2004, 126, 8256–8265. [Google Scholar] [CrossRef] [PubMed]
- Norel, L.; Rota, J.-B.; Chamoreau, L.-M.; Pilet, G.; Robert, V.; Train, C. Spin Transition and Exchange Interaction: Janus Visions of Supramolecular Spin Coupling between Face-to-Face Verdazyl Radicals. Angew. Chem. Int. Ed. 2011, 50, 7128–7131. [Google Scholar] [CrossRef] [PubMed]
- Balzani, V.; Credi, A.; Venturi, M. Molecular Devices and Machines—A Journey into the Nano World; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; ISBN 3527305068. [Google Scholar]
- Spruell, J.M.; Paxton, W.F.; Olsen, J.-C.; Benítez, D.; Tkatchouk, E.; Stern, C.L.; Trabolsi, A.; Friedman, D.C.; Goddard, W.A.; Stoddart, J.F. A Push-Button Molecular Switch. J. Am. Chem. Soc. 2009, 131, 11571–11580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, H.-R.; Vignon, S.A.; Celestre, P.C.; Perkins, J.; Jeppesen, J.O.; Di Fabio, A.; Ballardini, R.; Gandolfi, M.T.; Venturi, M.; Balzani, V.; et al. Redox-Controllable Amphiphilic [2] Rotaxanes. Chem. Eur. J. 2004, 10, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Biscarini, F.; Léon, S.; Zerbetto, F.; Bottari, G.; Leigh, D.A. Information Storage Using Supramolecular Surface Patterns. Science 2003, 299, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leigh, D.A.; Morales, M.Á.F.; Pérez, E.M.; Wong, J.K.Y.; Saiz, C.G.; Slawin, A.M.Z.; Carmichael, A.J.; Haddleton, D.M.; Brouwer, A.M.; Buma, W.J.; et al. Patterning through Controlled Submolecular Motion: Rotaxane-Based Switches and Logic Gates that Function in Solution and Polymer Films. Angew. Chem. Int. Ed. 2005, 44, 3062–3067. [Google Scholar] [CrossRef] [PubMed]
- Krober, J.; Codjovi, E.; Kahn, O.; Groliere, F.; Jay, C. A spin transition system with a thermal hysteresis at room temperature. J. Am. Chem. Soc. 1993, 115, 9810–9811. [Google Scholar] [CrossRef]
- Weber, B.; Bauer, W.; Obel, J. An Iron(II) Spin-Crossover Complex with a 70 K Wide Thermal Hysteresis Loop. Angew. Chem. Int. Ed. 2008, 47, 10098–10101. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. Spin-Crossover Materials; Halcrow, M.A., Ed.; John Wiley & Sons Ltd: Oxford, UK, 2013; ISBN 9781118519301. [Google Scholar]
- Gütlich, P.; Garcia, Y.; Spiering, H. Spin Transition Phenomena. In Magnetism: Molecules to Materials IV; Miller, J.S., Drillon, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 271–344. [Google Scholar]
- Gütlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- McGravey, J.J.; Lawthers, I. Photochemically-induced perturbation of the 1 A ⇌ 5 T equilibrium in Fe(II) complexes by pulsed laser irradiation in the metal-to-ligand charge-transfer absorption band. J. Chem. Soc. Chem. Commun. 1982, 906–907. [Google Scholar] [CrossRef]
- Hauser, A. Light-Induced Spin Crossover and the High-Spin → Low-Spin Relaxation. In Spin Crossover in Transition Metal Compounds II; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Bonhommeau, S.; Molnár, G.; Galet, A.; Zwick, A.; Real, J.-A.; McGarvey, J.J.; Bousseksou, A. One Shot Laser Pulse Induced Reversible Spin Transition in the Spin-Crossover Complex [Fe(C4H4N2){Pt(CN)4}] at Room Temperature. Angew. Chem. Int. Ed. 2005, 44, 4069–4073. [Google Scholar] [CrossRef] [PubMed]
- Bousseksou, A.; Negre, N.; Goiran, M.; Salmon, L.; Tuchagues, J.-P.; Boillot, M.-L.; Boukheddaden, K.; Varret, F. Dynamic triggering of a spin-transition by a pulsed magnetic field. Eur. Phys. J. B 2000, 13, 451–456. [Google Scholar] [CrossRef]
- Bousseksou, A.; Boukheddaden, K.; Goiran, M.; Consejo, C.; Boillot, M.-L.; Tuchagues, J.-P. Dynamic response of the spin-crossover solid Co(H2(fsa)2en)(py)2 to a pulsed magnetic field. Phys. Rev. B 2002, 65, 172412. [Google Scholar] [CrossRef]
- Ksenofontov, V.; Gaspar, A.B.; Gütlich, P. Pressure Effect Studies on Spin Crossover and Valence Tautomeric Systems. In Spin Crossover in Transition Metal Compounds III; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Niel, V.; Muñoz, M.C.; Gaspar, A.B.; Galet, A.; Levchenko, G.; Real, J.A. Thermal-, Pressure-, and Light-Induced Spin Transition in Novel Cyanide-Bridged FeII-AgI Bimetallic Compounds with Three-Dimensional Interpenetrating Double Structures {FeIILx[Ag(CN)2]2}⋅G. Chem. Eur. J. 2002, 8, 2446–2453. [Google Scholar] [CrossRef]
- Jeftić, J.; Hauser, A. Pressure Study of the Thermal Spin Transition and the High-Spin → Low-Spin Relaxation in the R-3 and P-1 Crystallographic Phases of [Zn1- xFex(ptz)6](BF4)2 Single Crystals (x = 0.1, 0.32, and 1; ptz = 1-n-propyltetrazole). J. Phys. Chem. B 1997, 101, 10262–10270. [Google Scholar] [CrossRef]
- Miyamachi, T.; Gruber, M.; Davesne, V.; Bowen, M.; Boukari, S.; Joly, L.; Scheurer, F.; Rogez, G.; Yamada, T.K.; Ohresser, P.; et al. Robust spin crossover and memristance across a single molecule. Nat. Commun. 2012, 3, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopakumar, T.G.; Matino, F.; Naggert, H.; Bannwarth, A.; Tuczek, F.; Berndt, R. Electron-Induced Spin Crossover of Single Molecules in a Bilayer on Gold. Angew. Chem. Int. Ed. 2012, 51, 6262–6266. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Goodwin, H.A. Spin Crossover—An Overall Perspective. In Spin Crossover in Transition Metal Compounds I; Gütlich, P., Goodwin, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Bousseksou, A.; McGarvey, J.J.; Varret, F.; Real, J.A.; Tuchagues, J.-P.; Dennis, A.C.; Boillot, M.L. Raman spectroscopy of the high- and low-spin states of the spin crossover complex Fe(phen)2(NCS)2: An initial approach to estimation of vibrational contributions to the associated entropy change. Chem. Phys. Lett. 2000, 318, 409–416. [Google Scholar] [CrossRef]
- Tayagaki, T.; Tanaka, K. Photoinduced Phase Transition to a New Macroscopic Spin-Crossover-Complex Phase. Phys. Rev. Lett. 2001, 86, 2886–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and Optical Switching of Iron(II) Complexes. Angew. Chem. Int. Ed. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Hauser, A.; Vef, A.; Adler, P. Intersystem crossing dynamics in Fe(II) coordination compounds. J. Chem. Phys. 1991, 95, 8710–8717. [Google Scholar] [CrossRef]
- Hauser, A. Intersystem crossing in Fe(II) coordination compounds. Coord. Chem. Rev. 1991, 111, 275–290. [Google Scholar] [CrossRef]
- Alam, M.S.; Stocker, M.; Gieb, K.; Müller, P.; Haryono, M.; Student, K.; Grohmann, A. Spin-State Patterns in Surface-Grafted Beads of Iron(II) Complexes. Angew. Chem. Int. Ed. 2010, 49, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Félix, G.; Abdul-Kader, K.; Mahfoud, T.; Gural’skiy, I.A.; Nicolazzi, W.; Salmon, L.; Molnár, G.; Bousseksou, A. Surface Plasmons Reveal Spin Crossover in Nanometric Layers. J. Am. Chem. Soc. 2011, 133, 15342–15345. [Google Scholar] [CrossRef] [PubMed]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M. Status and perspectives in thin films and patterning of spin crossover compounds. Phys. Chem. Chem. Phys. 2012, 14, 11867–11876. [Google Scholar] [CrossRef] [PubMed]
- Boldog, I.; Gaspar, A.B.; Martínez, V.; Pardo-Ibañez, P.; Ksenofontov, V.; Bhattacharjee, A.; Gütlich, P.; Real, J.A. Spin-Crossover Nanocrystals with Magnetic, Optical, and Structural Bistability Near Room Temperature. Angew. Chem. Int. Ed. 2008, 47, 6433–6437. [Google Scholar] [CrossRef] [PubMed]
- Volatron, F.; Catala, L.; Rivière, E.; Gloter, A.; Stéphan, O.; Mallah, T. Spin-Crossover Coordination Nanoparticles. Inorg. Chem. 2008, 47, 6584–6586. [Google Scholar] [CrossRef] [PubMed]
- Létard, J.-F.; Nguyen, O.; Daro, N. Nanoparticules d’un composé à transition de. spin Patent FR2894581, 2007. [Google Scholar]
- Coronado, E.; Galán-Mascarós, J.R.; Monrabal-Capilla, M.; García-Martínez, J.; Pardo-Ibáñez, P. Bistable Spin-Crossover Nanoparticles Showing Magnetic Thermal Hysteresis near Room Temperature. Adv. Mater. 2007, 19, 1359–1361. [Google Scholar] [CrossRef]
- Tokarev, A.; Salmon, L.; Guari, Y.; Nicolazzi, W.; Molnár, G.; Bousseksou, A. Cooperative spin crossover phenomena in [Fe(NH2trz)3](tosylate)2 nanoparticles. Chem. Commun. 2010, 46, 8011–8013. [Google Scholar] [CrossRef] [PubMed]
- Galán-Mascarós, J.R.; Coronado, E.; Forment-Aliaga, A.; Monrabal-Capilla, M.; Pinilla-Cienfuegos, E.; Ceolin, M. Tuning Size and Thermal Hysteresis in Bistable Spin Crossover Nanoparticles. Inorg. Chem. 2010, 49, 5706–5714. [Google Scholar] [CrossRef] [PubMed]
- Forestier, T.; Mornet, S.; Daro, N.; Nishihara, T.; Mouri, S.; Tanaka, K.; Fouché, O.; Freysz, E.; Létard, J.-F. Nanoparticles of iron(II) spin-crossover. Chem. Commun. 2008, 4327–4329. [Google Scholar] [CrossRef] [PubMed]
- Larionova, J.; Salmon, L.; Guari, Y.; Tokarev, A.; Molvinger, K.; Molnár, G.; Bousseksou, A. Towards the Ultimate Size Limit of the Memory Effect in Spin-Crossover Solids. Angew. Chem. Int. Ed. 2008, 47, 8236–8240. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, C.; Moitzi, C.; Schurtenberger, P.; Morgan, G.G.; Albrecht, M. Improved Cooperativity of Spin-Labile Iron(III) Centers by Self-Assembly in Solution. J. Am. Chem. Soc. 2008, 130, 14434–14435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandolfi, C.; Miyashita, N.; Kurth, D.G.; Martinho, P.N.; Morgan, G.G.; Albrecht, M. Organization of spin- and redox-labile metal centers into Langmuir and Langmuir–Blodgett films. Dalton Trans. 2010, 39, 4508–4516. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, C.; Morgan, G.G.; Albrecht, M. A magnetic iron(III) switch with controlled and adjustable thermal response for solution processing. Dalton Trans. 2012, 41, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Martinho, P.N.; Harding, C.J.; Müller-Bunz, H.; Albrecht, M.; Morgan, G.G. Inducing Spin Crossover in Amphiphilic Iron(III) Complexes. Eur. J. Inorg. Chem. 2010, 2010, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Martinho, P.N.; Ortin, Y.; Gildea, B.; Gandolfi, C.; McKerr, G.; O’Hagan, B.; Albrecht, M.; Morgan, G.G. Inducing hysteretic spin crossover in solution. Dalton Trans. 2012, 41, 7461–7463. [Google Scholar] [CrossRef] [PubMed]
- Martinho, P.N.; Lemma, T.; Gildea, B.; Picardi, G.; Müller-Bunz, H.; Forster, R.J.; Keyes, T.E.; Redmond, G.; Morgan, G.G. Template Assembly of Spin Crossover One-Dimensional Nanowires. Angew. Chem. Int. Ed. 2012, 51, 11995–11999. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, C.; Cotting, T.; Martinho, P.N.; Sereda, O.; Neels, A.; Morgan, G.G.; Albrecht, M. Synthesis and self-assembly of spin-labile and redox-active manganese(III) complexes. Dalton Trans. 2011, 40, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, K.B. Monomolecular Films of Fatty Acids on Glass. J. Am. Chem. Soc. 1934, 56, 495. [Google Scholar] [CrossRef]
- Blodgett, K.B.; Langmuir, I. Built-Up Films of Barium Stearate and Their Optical Properties. Phys. Rev. 1937, 51, 964–982. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, F.; Liu, T.; Yuan, M.; Wang, Z.-M.; Gao, S. Spin Crossover in a Series of Iron(II) Complexes of 2-(2-Alkyl-2H-tetrazol-5-yl)-1,10-phenanthroline: Effects of Alkyl Side Chain, Solvent, and Anion. Inorg. Chem. 2007, 46, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Hayami, S.; Danjobara, K.; Shigeyoshi, Y.; Inoue, K.; Ogawa, Y.; Maeda, Y. Crystal structure and mesogenic property of an iron(II) complex with a terpyridine derivative ligand. Inorg. Chem. Commun. 2005, 8, 506–509. [Google Scholar] [CrossRef]
- Scott, H.S.; Moubaraki, B.; Paradis, N.; Chastanet, G.; Létard, J.-F.; Batten, S.R.; Murray, K.S. 2,2′-Dipyridylamino-based ligands with substituted alkyl chain groups and their mononuclear-M(II) spin crossover complexes. J. Mater. Chem. C 2015, 3, 7845–7857. [Google Scholar] [CrossRef]
- Ueno, S.; Kawasaki, T.; Okabayashi, J.; Kitazawa, T. 2D Spin-Crossover Coordination Polymer Fe(hexyl-nicotinate)2[Au(CN)2]2. Bull. Chem. Soc. Jpn. 2016, 89, 581–583. [Google Scholar] [CrossRef]
- Seredyuk, M.; Gaspar, A.B.; Ksenofontov, V.; Galyametdinov, Y.; Kusz, J.; Gütlich, P. Does the Solid−Liquid Crystal Phase Transition Provoke the Spin-State Change in Spin-Crossover Metallomesogens? J. Am. Chem. Soc. 2008, 130, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Seredyuk, M. Iron(II) metallomesogens based on symmetrical tripod ligands. Inorg. Chim. Acta 2012, 380, 65–71. [Google Scholar] [CrossRef]
- Seredyuk, M.; Gaspar, A.B.; Ksenofontov, V.; Galyametdinov, Y.; Kusz, J.; Gütlich, P. Iron(II) Metallomesogens Exhibiting Coupled Spin State and Liquid Crystal Phase Transitions near Room Temperature. Adv. Funct. Mater. 2008, 18, 2089–2101. [Google Scholar] [CrossRef]
- Vologzhanina, A.V.; Belov, A.S.; Novikov, V.V.; Dolganov, A.V.; Romanenko, G.V.; Ovcharenko, V.I.; Korlyukov, A.A.; Buzin, M.I.; Voloshin, Y.Z. Synthesis and Temperature-Induced Structural Phase and Spin Transitions in Hexadecylboron-Capped Cobalt(II) Hexachloroclathrochelate and Its Diamagnetic Iron(II)-Encapsulating Analogue. Inorg. Chem. 2015, 54, 5827–5838. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, Y.Z.; Varzatskii, O.A.; Stash, A.I.; Belsky, V.K.; Bubnov, Y.N.; Vorontsov, I.I.; Potekhin, K.A.; Antipin, M.Y.; Polshin, E.V. Template synthesis, structure and unusual series of phase transitions in clathrochelate iron(II) α-dioximates and oximehydrazonates formed by capping with functionalized boron-containing agents. Polyhedron 2001, 20, 2721–2733. [Google Scholar] [CrossRef]
- Han, W.-K.; Li, Z.-H.; Zhu, W.; Li, T.; Li, Z.; Ren, X.; Gu, Z.-G. Molecular isomerism induced Fe(II) spin state difference based on the tautomerization of the 4(5)-methylimidazole group. Dalton Trans. 2017, 46, 4218–4224. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, J.A.; White, N.G.; Jameson, G.N.L.; Tallon, J.L.; Brooker, S. Effect of Counteranion X on the Spin Crossover Properties of a Family of Diiron(II) Triazole Complexes [FeII2(PMAT)2](X)4. Inorg. Chem. 2011, 50, 4586–4597. [Google Scholar] [CrossRef] [PubMed]
- Schlamp, S.; Weber, B.; Naik, A.D.; Garcia, Y. Cooperative spin transition in a lipid layer like system. Chem. Commun. 2011, 47, 7152–7154. [Google Scholar] [CrossRef] [PubMed]
- Schlamp, S.; Thoma, P.; Weber, B. New Octahedral, Head-Tail Iron(II) Complexes with Spin Crossover Properties. Eur. J. Inorg. Chem. 2012, 2012, 2759–2768. [Google Scholar] [CrossRef]
- Schlamp, S.; Dankhoff, K.; Weber, B. Amphiphilic iron(II) complexes with short alkyl chains – crystal packing and spin transition properties. N. J. Chem. 2014, 38, 1965–1972. [Google Scholar] [CrossRef]
- Schlamp, S.; Lochenie, C.; Bauer, T.; Kempe, R.; Weber, B. Iron(II) Spin-Crossover Complexes with Schiff Base Like Ligands and N-Alkylimidazoles. Eur. J. Inorg. Chem. 2015, 2015, 408–413. [Google Scholar] [CrossRef]
- Schlamp, S.; Thoma, P.; Weber, B. Influence of the Alkyl Chain Length on the Self-Assembly of Amphiphilic Iron Complexes: An Analysis of X-ray Structures. Chem. Eur. J. 2014, 20, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Shimayama, K.; Takami, K.; Hirata, K.; Amolegbe, S.; Nakamura, M.; Lindoy, L.; Hayami, S. Structures and Magnetic Properties of Iron(III) Complexes with Long Alkyl Chains. Crystals 2014, 4, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Rosario-Amorin, D.; Dechambenoit, P.; Bentaleb, A.; Rouzières, M.; Mathonière, C.; Clérac, R. Multistability at Room Temperature in a Bent-Shaped Spin-Crossover Complex Decorated with Long Alkyl Chains. J. Am. Chem. Soc. 2018, 140, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Hayami, S.; Shigeyoshi, Y.; Akita, M.; Inoue, K.; Kato, K.; Osaka, K.; Takata, M.; Kawajiri, R.; Mitani, T.; Maeda, Y. Reverse Spin Transition Triggered by a Structural Phase Transition. Angew. Chem. Int. Ed. 2005, 44, 4899–4903. [Google Scholar] [CrossRef] [PubMed]
- Talham, D.R. Conducting and Magnetic Langmuir−Blodgett Films. Chem. Rev. 2004, 104, 5479–5502. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.B.; Seredyuk, M. Spin crossover in soft matter. Coord. Chem. Rev. 2014, 268, 41–58. [Google Scholar] [CrossRef]
- Ruaudel-Teixier, A.; Barraud, A.; Coronel, P.; Kahn, O. Spin transition in a magnetic Langmuir-Blodgett film. Thin Solid Films 1988, 160, 107–115. [Google Scholar] [CrossRef]
- Roubeau, O.; Agricole, B.; Clérac, R.; Ravaine, S. Triazole-Based Magnetic Langmuir−Blodgett Films: Paramagnetic to Spin-Crossover Behavior. J. Phys. Chem. B 2004, 108, 15110–15116. [Google Scholar] [CrossRef]
- Soyer, H.; Dupart, E.; Gómez-García, C.J.; Mingotaud, C.; Delhaès, P. First Magnetic Observation of a Spin Crossover in a Langmuir-Blodgett Film. Adv. Mater. 1999, 11, 382–384. [Google Scholar] [CrossRef]
- Létard, J.F.; Nguyen, O.; Soyer, H.; Mingotaud, C.; Delhaès, P.; Kahn, O. First Evidence of the LIESST Effect in a Langmuir−Blodgett Film. Inorg. Chem. 1999, 38, 3020–3021. [Google Scholar] [CrossRef]
- Bodenthin, Y.; Pietsch, U.; Möhwald, H.; Kurth, D.G. Inducing Spin Crossover in Metallo-supramolecular Polyelectrolytes through an Amphiphilic Phase Transition. J. Am. Chem. Soc. 2005, 127, 3110–3114. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, J.A.; White, N.G.; Gandolfi, C.; Albrecht, M.; Jameson, G.N.L.; Tallon, J.L.; Brooker, S. Room-temperature spin crossover and Langmuir–Blodgett film formation of an iron(II) triazole complex featuring a long alkyl chain substituent: The tail that wags the dog. Chem. Commun. 2010, 46, 6464–6466. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayami, S. Amphiphilic and Liquid Crystalline Spin-Crossover Complexes. In Spin-Crossover Materials; John Wiley & Sons Ltd: Oxford, UK, 2013; pp. 321–345. [Google Scholar]
- Akiyoshi, R.; Kuroiwa, K.; Alao Amolegbe, S.; Nakaya, M.; Ohtani, R.; Nakamura, M.; Lindoy, L.F.; Hayami, S. Supramolecular architectures self-assembled using long chain alkylated spin crossover cobalt(II) compounds. Chem. Commun. 2017, 53, 4685–4687. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.L.C.; Johnson, C.; Elliott, A.B.S.; Gordon, K.C.; Albrecht, M.; Brooker, S. “Tail” Tuning of Iron(II) Spin Crossover Temperature by 100 K. Inorg. Chem. 2015, 54, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.J.; Martinho, P.N.; Gildea, B.J.; Holbrey, J.D.; Morgan, G.G. Robust Room Temperature Hysteresis in an Fe(III) Spin Crossover Metallomesogen. Eur. J. Inorg. Chem. 2016, 2016, 2025–2029. [Google Scholar] [CrossRef] [Green Version]
- Tweedle, M.F.; Wilson, L.J. Variable spin iron(III) chelates with hexadentate ligands derived from triethylenetetramine and various salicylaldehydes. Synthesis, characterization, and solution state studies of a new 2T ⇌ 6A spin equilibrium system. J. Am. Chem. Soc. 1976, 98, 4824–4834. [Google Scholar] [CrossRef]
- Carrano, C.J.; Carrano, M.W.; Sharma, K.; Backes, G.; Sanders-Loehr, J. Resonance Raman spectra of high- and low-spin ferric phenolates. Models for dioxygenases and nitrile hydratase. Inorg. Chem. 1990, 29, 1865–1870. [Google Scholar] [CrossRef]











| Concentration (mol L−1) | Diameter (nm) | Polydispersity |
|---|---|---|
| 5.70 × 10−4 | 400 | 0.310 |
| 330 | 0.235 | |
| 320 | 0.102 | |
| 2.85 × 10−4 | 300 | 0.364 |
| 270 | 0.201 | |
| 270 | 0.378 | |
| 1.43 × 10−4 | 320 | 0.333 |
| 250 | 0.051 | |
| 280 | 0.318 | |
| 7.13 × 10−5 | 300 | 0.283 |
| 320 | 0.165 | |
| 290 | 0.218 |
| Bond Type | rRaman Shift (cm−1) LL = 532 nm | Raman Shift (cm−1) LL = 785 nm | Literature [88] | IR (cm−1) | |||
|---|---|---|---|---|---|---|---|
| complex 1 | T = 295 K | T = 79 K | T = 295 K | T = 79 K | HS | LS | - |
| υ(Fe–O) | 609 | 611 | 611 | 613,623 | 612 | 622 | 602 |
| υ(C–O) | 1311 | 1310 | 1309 | 1308 | 1310 | 1310 | 1302 |
| υ(C=C) | 1596 | 1598 | 1599 | 1600 | 1600 | 1600 | |
| υ(C=N) | 1620 | 1622 | 1619 | 1625 | 1630 | absent | 1620 |
| complex 2 | T = 295 K | T = 79 K | T = 295 K | T = 79 K | HS | LS | - |
| υ(Fe–O) | 615,618 | 618 | 613 | 613,623 | 612 | 622 | 602 |
| υ(C–O) | 1317 | 1317 | 1317 | 1310 | 1310 | 1310 | 1302 |
| υ(C=C) | 1603 | 1607 | 1602 | 1605 | 1600 | 1600 | - |
| υ(C=N) | 1627 | 1635 | 1627 | absent | 1630 | absent | 1620 |
| complex 3 | T = 295 K | T = 79 K | T = 295 K | T = 79 K | HS | LS | - |
| υ(Fe–O) | 611,614 | 612,614 | 612,625 | 613,623 | 612 | 622 | 602 |
| υ(C–O) | 1308 | 1307 | 1309 | 1310 | 1310 | 1310 | 1302 |
| υ(C=C) | 1599 | 1601 | 1600 | 1605 | 1600 | 1600 | - |
| υ(C=N) | 1627 | 1630 | 1627 | absent | 1630 | absent | 1620 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinho, P.N.; Kühne, I.A.; Gildea, B.; McKerr, G.; O’Hagan, B.; Keyes, T.E.; Lemma, T.; Gandolfi, C.; Albrecht, M.; Morgan, G.G. Self-Assembly Properties of Amphiphilic Iron(III) Spin Crossover Complexes in Water and at the Air–Water Interface. Magnetochemistry 2018, 4, 49. https://doi.org/10.3390/magnetochemistry4040049
Martinho PN, Kühne IA, Gildea B, McKerr G, O’Hagan B, Keyes TE, Lemma T, Gandolfi C, Albrecht M, Morgan GG. Self-Assembly Properties of Amphiphilic Iron(III) Spin Crossover Complexes in Water and at the Air–Water Interface. Magnetochemistry. 2018; 4(4):49. https://doi.org/10.3390/magnetochemistry4040049
Chicago/Turabian StyleMartinho, Paulo N., Irina A. Kühne, Brendan Gildea, George McKerr, Barry O’Hagan, Tia E. Keyes, Tibebe Lemma, Claudio Gandolfi, Martin Albrecht, and Grace G. Morgan. 2018. "Self-Assembly Properties of Amphiphilic Iron(III) Spin Crossover Complexes in Water and at the Air–Water Interface" Magnetochemistry 4, no. 4: 49. https://doi.org/10.3390/magnetochemistry4040049