Short-Term Traffic-Related Exposures and Biomarkers of Nitro-PAH Exposure and Oxidative DNA Damage
Abstract
:1. Introduction
2. Methodology
2.1. Study Population
2.2. Microenvironment Exposure Measurements
2.3. Biomarker Sampling and Analysis
2.4. Health and Personal Habit Assessment
2.5. Statistical Analyses
- Yijk: biomarker levels for subject i, on day j, at the k (pre-or post-shift) measurement point;
- Expi: entered as the average of the daily pollutant levels on the two measured (first and last-day) work-shifts;
- dayj: measurement day j: first or last day worked during the week;
- shiftk: measurement time k relevant to shift, pre- or post-shift;
- covariatesijk: covariates entered in the model as listed above;
- bi: random intercept for each individual i;
- eijk: within subject error.
3. Results
3.1. Study Population
| Characteristic | Total |
|---|---|
| Total no. | 82 |
| Race, White (no. (%)) | 76 (93%) |
| Race, Non-white (no. (%)) | 6 (7%) |
| Age (years, mean ± SD) | 50.2 ± 8.6 |
| BMI (kg/m2, mean ± SD) | 30.1 ± 4.5 |
| Current smoker (no. (%)) | 13 (16%) |
| Past smoker (no. (%)) | 37 (45%) |
| Cigs smoked per shift (mean ± SD) | 6.9 ± 5.8 |
| Grilled/smoked food consumption (no. (%)) | 38 (46) |
| Chronic respiratory disease (no. (%)) | 14 (17) |
| Total workdays (mean ± SD) | 4.0 ± 0.5 |
| Average shift duration (h, mean ± SD) | 9.5 ± 1.9 |
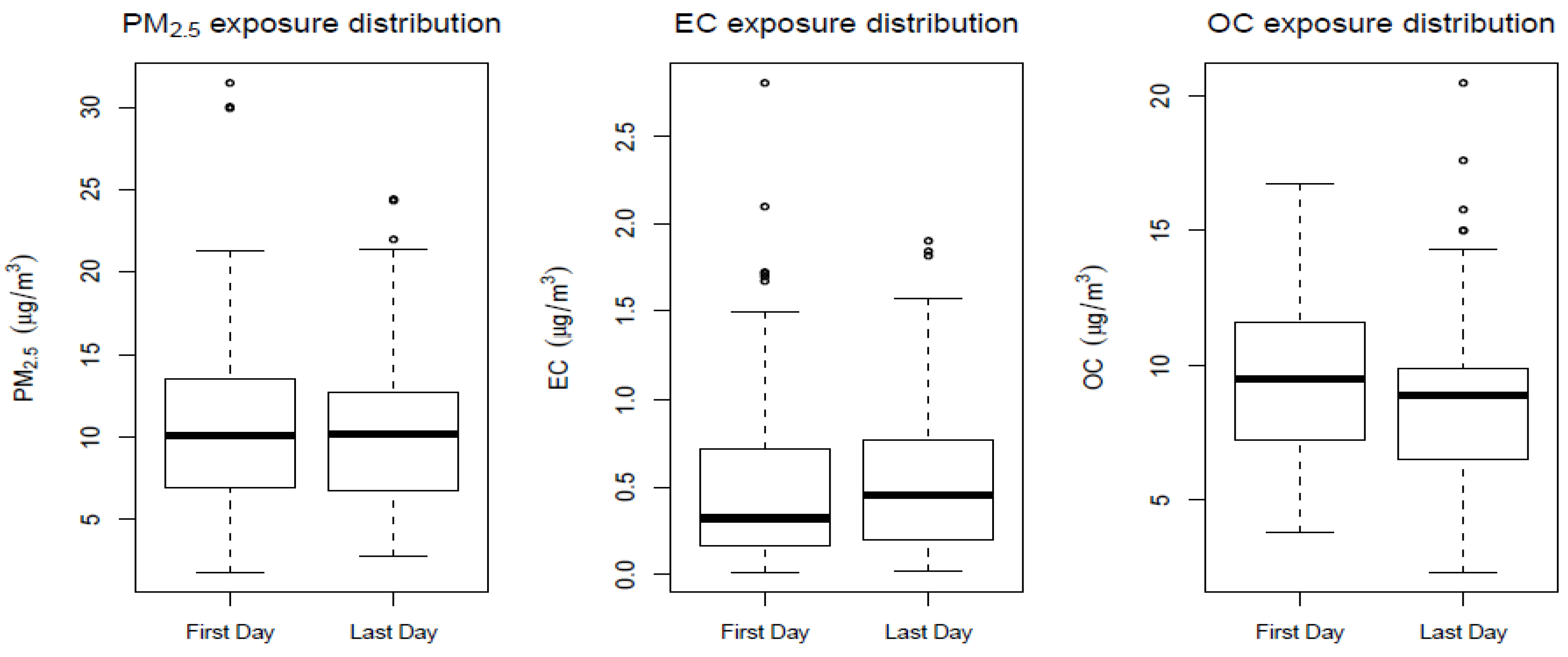
3.2. Pollutant Exposure Measurements
3.3. Associations between Pollutant Measurements and Biomarkers

| Exposure | IQR | 1 & 2-AN change (95% CI) | 1-AP change (95% CI) |
|---|---|---|---|
| First-Last day mean b | |||
| PM2.5 (μg/m3) | 5.54 | 141.8 (53.3, 230.2) | 2.4 (−32.2, 37.1) |
| EC (μg/m3) | 0.51 | 64.7 (−32.2, 161.7) | 18.3 (−17.5, 54.2) |
| OC (μg/m3) | 3.97 | −49.8 (−154.3, 54.7) | 14.3 (−24.2, 52.9) |
| Daily c | |||
| PM2.5 (μg/m3) | 6.20 | 65.2 (−40.6, 171.0) | −23.7 (−58.9, 11.4) |
| EC (μg/m3) | 0.55 | 105.9 (−3.8, 215.7) | −9.7 (−41.8, 22.4) |
| OC (μg/m3) | 3.89 | −36.1 (−145.1, 72.8) | 2.9 (−30.0, 35.8) |
| Exposure b | IQR | All Participants Change in 8-OHdG (95% CI) | Non-Smokers Change in 8-OHdG, (95% CI) |
|---|---|---|---|
| PM2.5 (μg/m3) | 5.54 | 5.50 (−2.50, 13.50) | 8.54 (−0.98, 18.05) |
| EC (μg/m3) | 0.51 | 2.36 (−6.45, 11.18) | 3.10 (−6.77, 12.97) |
| OC (μg/m3) | 3.97 | 1.08 (−8.08, 10.24) | 2.40 (−8.91, 13.70) |
| 1 & 2-AN (pg/mg creatinine) | 242.85 | 2.38 (0.19, 4.58) | 1.54 (−1.22, 4.30) |
| 1-AP (pg/mg creatinine) | 50.19 | 1.18 (−0.33, 2.68) | 1.35 (−0.26, 2.95) |
4. Discussion
5. Conclusions
Abbreviations
| 1 & 2-AN | 1-aminonaphthalene and 2-aminonaphthalene |
| 1-AP | 1-aminopyrene |
| 1 & 2-NN | 1-nitronaphthalene and 2-nitronaphthalene |
| 1-NP | 1-nitropyrenre |
| 8-OHdG | 8-hydroxy-2'-deoxyguanosine |
| BMI | body mass index |
| EC | elemental carbon |
| IQR | interquartile range |
| LOD | level of detection |
| OC | organic carbon |
| PAH | polycyclic aromatic hydrocarbon |
| PM | particulate matter |
| PM1.0 | particulate matter less than 1.0 µm in diameter |
| PM2.5 | particulate matter less than 2.5 µm in diameter |
| SD | standard deviation |
| SHS | second-hand smoke |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Dockery, D.W.; Luttmann-Gibson, H.; Rich, D.Q.; Link, M.S.; Mittleman, M.A.; Gold, D.R.; Koutrakis, P.; Schwartz, J.D.; Verrier, R.L. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ. Health Perspect. 2005, 113, 670–674. [Google Scholar] [CrossRef]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermont, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Brauer, M.; Hoeck, G.; Smit, H.A.; de Jongste, J.C.; Gerritsen, J.; Postma, D.S.; Kerkhof, M.; Brunekreef, B. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur. Respir. J. 2007, 29, 879–888. [Google Scholar] [CrossRef]
- IARC: Diesel engine exhaust carcinogenic. Available online: http://w2.iarc.fr/en/media-centre/pr/2012/ (accessed on 16 December 2013).
- IARC: Outdoor air pollution a leading environmental cause of cancer deaths. Available online: http://www.iarc.fr/en/media-centre/iarcnews/pdf/pr221_E.pdf (accessed on 16 December 2013).
- Dockery, D.W.; Pope, A.C., III; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D.W. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am. J. Respir. Crit. Care Med. 2006, 173, 667–672. [Google Scholar] [CrossRef]
- Paustenbach, D.; Galbraith, D. Biomonitoring and biomarkers: Exposure assessment will never be the same. Environ. Health Perspect. 2006, 114, 1143–1149. [Google Scholar] [CrossRef]
- Lewtas, J. Air pollution combustion emissions: Characterization of causative agents and mechanisms associated with cancer, reproductive and cardiovascular effects. Mut. Res. 2007, 636, 95–133. [Google Scholar] [CrossRef]
- Vineis, P.; Husgafvel-Pursiainen, K. Air pollution and cancer: Biomarker studies in human populations. Carcinogenesis 2005, 26, 1846–1855. [Google Scholar] [CrossRef]
- Demetriou, C.A.; Raaschou-Nielsen, O.; Loft, S.; Moller, P.; Vermeulen, R.; Palli, D.; Chadeau-Hyam, M.; Xun, W.W.; Vineis, P. Biomarkers of ambient air pollution and lung cancer: A systematic review. Occup. Environ. Med. 2012, 69, 619–627. [Google Scholar] [CrossRef]
- Zwirner-Baier, I.; Neumann, H.G. Polycyclic nitroarenes (nitro-PAHs) as biomarkers of exposure to diesel exhaust. Mutat. Res. 1999, 441, 135–144. [Google Scholar] [CrossRef]
- Seidel, A.; Dahmann, D.; Krekeler, H.; Jacob, J. Biomonitoring of polycyclic aromatic compounds in the urine of mining workers occupationally exposed to diesel exhaust. Int. J. Hyg. Environ. Health 2002, 204, 333–338. [Google Scholar] [CrossRef]
- Huyck, S.; Ohman-Strickland, P.; Zhang, L.; Tong, J.; Xu, X.; Zhang, J. Determining times to maximum urine excretion of 1-aminopyrene after diesel exhaust exposure. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 650–655. [Google Scholar] [CrossRef]
- Bigert, C.; Gustavsson, P.; Hallqvist, J.; Hogstedt, C.; Lewne, M.; Plato, N.; Reuterwall, C.; Schéele, P. Myocardial infarction among professional drivers. Epidemiology 2003, 14, 333–339. [Google Scholar]
- Gustavsson, P.; Plato, N.; Hallqvist, J.; Hogstedt, C.; Lewne, M.; Reuterwall, C.; Schéele, P. A population-based case-referent study of myocardial infarction and occupational exposure to motor exhaust, other combustion products, organic solvents, lead, and dynamite. Stockholm Heart Epidemiology Program (SHEEP) Study Group. Epidemiology 2001, 12, 222–228. [Google Scholar] [CrossRef]
- Vermeulen, R.; Silverman, D.T.; Garshick, E.; Vlaanderen, J.; Portengen, L.; Steenland, K. Exposure-response estimates dor diesel exhaust and lung cancer mortality based on data from three occupational cohorts. Environ. Health Perspect. 2014, 122, 172–177. [Google Scholar]
- Hart, J.E.; Garshick, E.; Smith, T.J.; Davis, M.E.; Laden, F. Ischaemic heart disease mortality and years of work in trucking industry workers. Occup. Environ. Med. 2013, 70, 523–528. [Google Scholar] [CrossRef]
- Garshick, E.; Laden, F.; Hart, J.E.; Davis, M.; Eisen, E.A.; Smith, T.J. Lung cancer and elemental carbon exposure in trucking industry workers. Environ. Health Perspect. 2012, 120, 1301–1306. [Google Scholar] [CrossRef]
- Albinet, A.; Leoz-Garziandia, E.; Budzinski, H.; Villenave, E. Polycyclic aromatic hydrocarbons (PAHs), nitrated PAHs and oxygenated PAHs in ambient air of the Marseilles area (South of France): Concentrations and sources. Sci. Total Environ. 2007, 384, 280–292. [Google Scholar] [CrossRef]
- Reisen, F.; Arey, J. Atmospheric reactions influence seasonal PAH and nitro-PAH concentrations in the Los Angeles Basin. Environ. Sci. Technol. 2005, 39, 64–73. [Google Scholar] [CrossRef]
- Bamford, H.A.; Bezabeh, D.Z.; Schantz, S.; Wise, S.A.; Baker, J.E. Determination and comparison of nitrated-polycyclic aromatic hydrocarbons measured in air and diesel particulate reference materials. Chemosphere 2003, 50, 575–87. [Google Scholar] [CrossRef]
- Lagorio, S.; Tagesson, C.; Forastiere, F.; Iavarone, I.; Axelson, O.; Carer, A. Exposure to benzene and urinary concentrations of 8-hydroxydeoxyguanosine, a biological marker of oxidative damage to DNA. Occup. Environ. Med. 1994, 51, 739–743. [Google Scholar] [CrossRef]
- Ren, C.; Fang, S.; Wright, R.O.; Suh, H.; Schwartz, J. Urinary 8-hydroxy-2'-deoxyguanosine as a biomarker of DNA damage induced by ambient pollution in the normative aging study. Occup. Environ. Med. 2010, 68, 562–569. [Google Scholar]
- Wei, Y.; Han, I.K.; Shao, M.; Hu, M.; Zhang, O.J.; Tang, X. PM2.5 constituents and oxidative DNA damage in humans. Environ. Sci. Technol. 2009, 43, 4757–4762. [Google Scholar]
- Neophytou, A.M.; Hart, J.E.; Cavallari, J.M.; Smith, T.J.; Dockery, D.W.; Coull, B.A.; Garshick, E.; Laden, F. Traffic-related exposures and biomarkers of systemic inflammation, endothelial activation and oxidative stress: A panel study in the US trucking industry. Environ. Health 2013, 12, 105. [Google Scholar] [CrossRef]
- NIOSH. Elemental Carbon (Diesel Particulate) 5040. In NIOSH Manual of Analytical Methods, 4th Ed. edU.S Department of Helath and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH): Cincinati, OH, USA, 2003. [Google Scholar]
- Tainio, M.; Tuomisto, J.T.; Hanninen, O.; Aarnio, P.; Koistinen, K.J.; Jantunen, M.J.; Pekkanen, J. Health effects caused by primary fine particulate matter (PM2.5) emitted from buses in the Helsinki metropolitan area, Finland. Risk. Anal. 2005, 25, 151–160. [Google Scholar]
- Yanosky, J.D.; MacIntosh, D.L. A comparison of four gravimetric fine particle sampling methods. J. Air Waste Manag. Assoc. 2001, 51, 878–884. [Google Scholar] [CrossRef]
- Laumbach, R.; Tong, J.; Zhang, L.; Ohman-Strickland, P.; Stern, A.; Fiedler, N.; Kipen, H.; Kelly-McNeil, K.; Lioy, P.; Zhang, J. Quantification of 1-aminopyrene in human urine after a controlled exposure to diesel exhaust. J. Environ. Monit. 2009, 11, 153–159. [Google Scholar] [CrossRef]
- Huang, W.; Wang, G.; Lu, S.E.; Kipen, H.M.; Wang, Y.; Hu, M.; Lin, W.; Rich, D.Q.; Ohman-Strickland, P.; Diehl, S.R.; et al. Inflammatory and Oxidative Stress Responses of Healthy Young Adults to Changes in Air Quality during the Beijing Olympics. Am. J. Respir. Crit. Care Med. 2012, 186, 1150–1159. [Google Scholar] [CrossRef]
- Ferris, B.G. Epidemiology Stamdardization Project (American Thoracic Society). Am. Rev. Respir. Dis. 1978, 118, 1–120. [Google Scholar]
- Igishi, T.; Hitsuda, Y.; Kato, K.; Sako, T.; Burioka, N.; Yasuda, K.; Sano, H.; Shigeoka, Y.; Nakanishi, H.; Shimizu, E. Elevated urinary 8-hydroxydeoxyguanosine, a biomarker of oxidative stress, and lack of association with antioxidant vitamins in chronic obstructive pulmonary disease. Respirology 2003, 8, 455–460. [Google Scholar] [CrossRef]
- Scherer, G. Biomonitoring of inhaled complex mixtures—Ambient air, diesel exhaust and cigarette smoke. Exp. Toxicol. Pathol. 2005, 57, 75–110. [Google Scholar] [CrossRef]
- Riffelmann, M.; Muller, G.; Schmieding, W.; Popp, W; Norpoth, K. Biomonitoring of urinary aromatic amines and arylamine hemoglobin adducts in exposed workers and nonexposed control persons. Int. Arch. Occup. Environ. Health 1995, 68, 36–43. [Google Scholar]
- Riedel, K.; Scherer, G.; Engl, J.; Heinz-Werner, H.; Tricker, A.R. Determination of three carcinogenic aromatic amines in urine of smokers and nonsmokers. J. Anal. Toxicol. 2006, 30, 187–195. [Google Scholar] [CrossRef]
- Lindner, D.; Smith, S.; Leroy, C.M.; Tricker, A.M. Comparison of exposure to selected cigarette smoke constituents in adult smokers and nonsmokers in a European, multicenter, observational study. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1524–1536. [Google Scholar] [CrossRef]
- Miller-Schulze, J.P.; Paulsen, M.; Kameda, T.; Toriba, A.; Tang, N.; Tamura, K.; Dong, L.; Zhang, X.; Hayakawa, K.; Yost, M.G.; et al. Evaluations of urinary metabolites of 1-nitropyrene as biomarkers for exposure to diesel exhaust in taxi drivers of Shenyang, China. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 170–175. [Google Scholar] [CrossRef]
- Wang, Y.; Hopke, P.K.; Rattigan, O.V.; Chalupa, D.C.; Utell, M.J. Multiple-year black carbon measurements and source apportionment using δ-C in Rochester, New York. J. Air Waste Manag. Assoc. 2012, 62, 880–887. [Google Scholar] [CrossRef]
- Davis, M.E.; Hart, J.E.; Laden, F.; Garshick, E.; Smith, T.J. A retrospective assessment of occupational exposure to elemental carbon in the U.S. trucking industry. Environ. Health Perspect. 2011, 119, 997–1002. [Google Scholar]
- Smith, T.J.; Davis, M.E.; Reaser, P.; Natkin, J.; Hart, J.E.; Laden, F.; Heft, A.; Garshick, E. Overview of particulate exposures in the US trucking industry. J. Environ. Monit. 2006, 8, 711–720. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Neophytou, A.M.; Hart, J.E.; Chang, Y.; Zhang, J.; Smith, T.J.; Garshick, E.; Laden, F. Short-Term Traffic-Related Exposures and Biomarkers of Nitro-PAH Exposure and Oxidative DNA Damage. Toxics 2014, 2, 377-390. https://doi.org/10.3390/toxics2030377
Neophytou AM, Hart JE, Chang Y, Zhang J, Smith TJ, Garshick E, Laden F. Short-Term Traffic-Related Exposures and Biomarkers of Nitro-PAH Exposure and Oxidative DNA Damage. Toxics. 2014; 2(3):377-390. https://doi.org/10.3390/toxics2030377
Chicago/Turabian StyleNeophytou, Andreas M., Jaime E. Hart, Yan Chang, Junfeng Zhang, Thomas J. Smith, Eric Garshick, and Francine Laden. 2014. "Short-Term Traffic-Related Exposures and Biomarkers of Nitro-PAH Exposure and Oxidative DNA Damage" Toxics 2, no. 3: 377-390. https://doi.org/10.3390/toxics2030377
APA StyleNeophytou, A. M., Hart, J. E., Chang, Y., Zhang, J., Smith, T. J., Garshick, E., & Laden, F. (2014). Short-Term Traffic-Related Exposures and Biomarkers of Nitro-PAH Exposure and Oxidative DNA Damage. Toxics, 2(3), 377-390. https://doi.org/10.3390/toxics2030377




