Effects of Two Doses of Curry Prepared with Mixed Spices on Postprandial Ghrelin and Subjective Appetite Responses—A Randomized Controlled Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
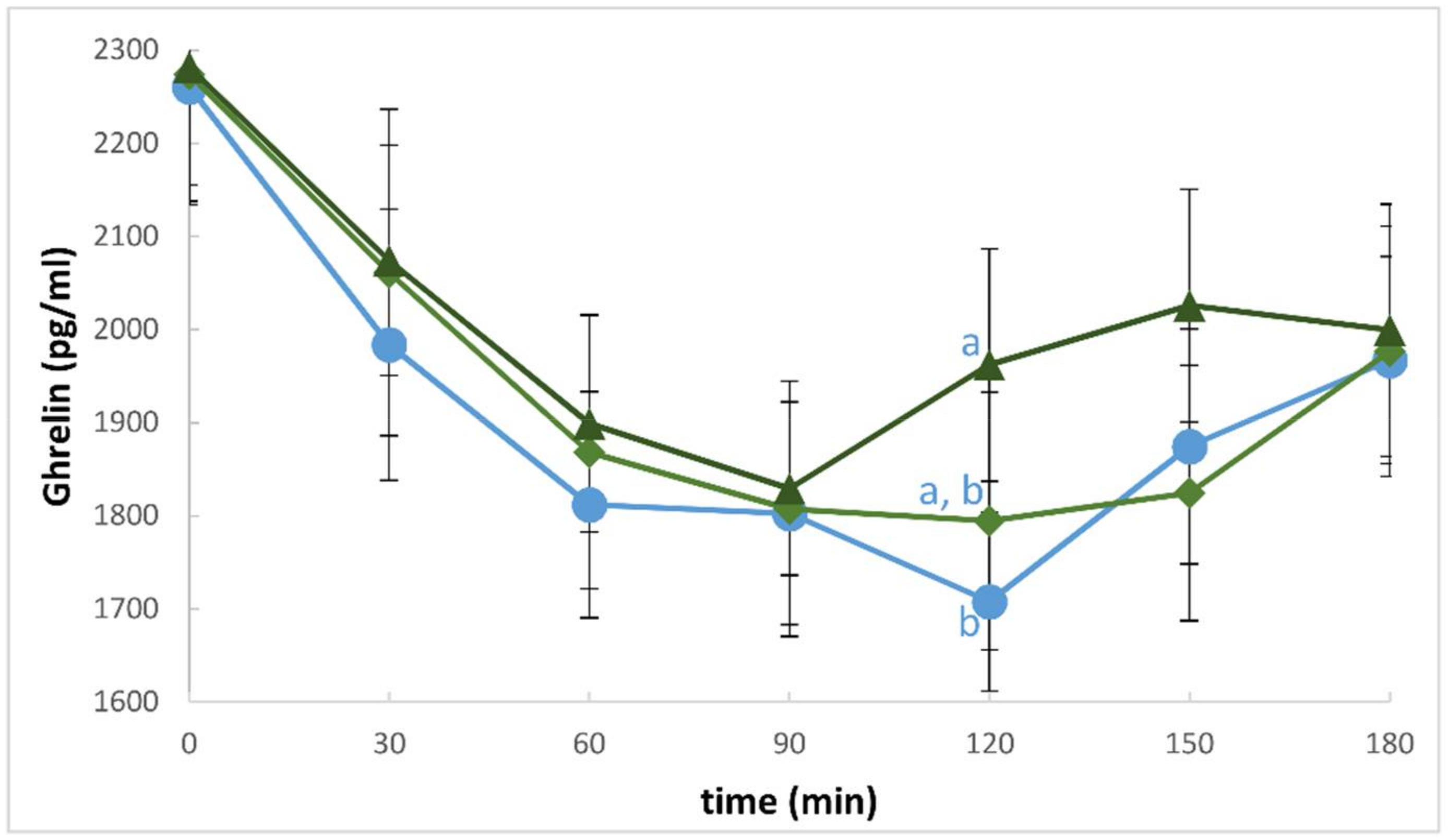
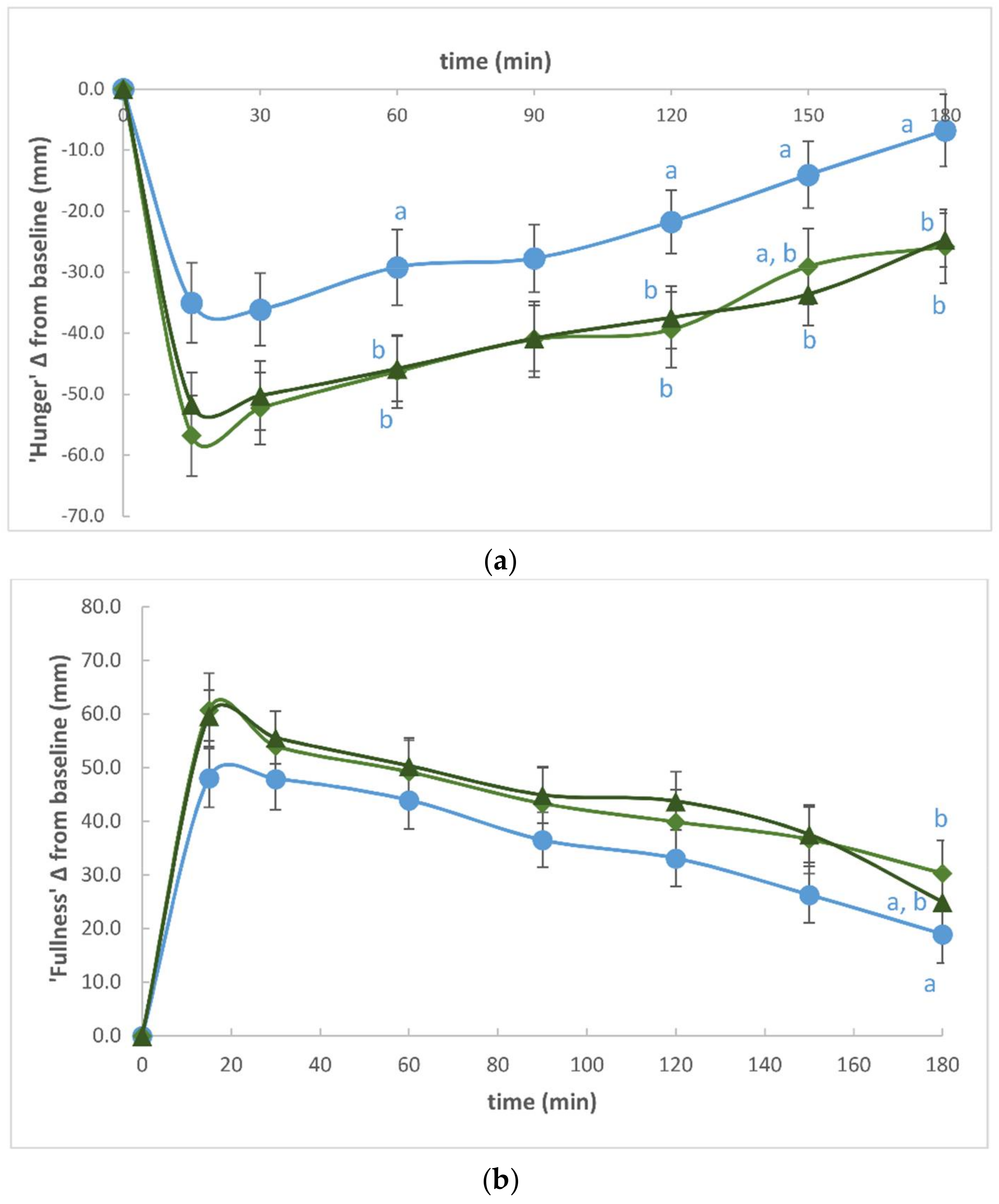
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mattes, R.D. Spices and energy balance. Physiol. Behav. 2012, 107, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Warwick, Z.S.; Hall, W.G.; Pappas, T.N.; Schiffman, S.S. Taste and smell sensations enhance the satiating effect of both a high-carbohydrate and a high-fat meal in humans. Physiol. Behav. 1993, 53, 553–563. [Google Scholar] [CrossRef]
- Masic, U.; Yeomans, M.R. Does monosodium glutamate interact with macronutrient composition to influence subsequent appetite? Physiol. Behav. 2013, 116–117, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.B.; Møller, P.; Flint, A.; Martens, M.; Raben, A. Effect of sensory perception of foods on appetite and food intake: A review of studies on humans. Int. J. Obes. 2003, 27. [Google Scholar] [CrossRef] [PubMed]
- McCrickerd, K.; Forde, C.G. Sensory influences on food intake control: Moving beyond palatability. Obes. Rev. 2016, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.C.; Polsky, S.; Stark, R.; Zhaoxing, P.; Hill, J.O. The influence of herbs and spices on overall liking of reduced fat food. Appetite 2014, 79, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Manero, J.; Phillips, C.; Ellison, B.; Lee, S.-Y.; Nickols-Richardson, S.M.; Chapman-Novakofski, K.M. Influence of seasoning on vegetable selection, liking and intent to purchase. Appetite 2017, 116, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, N.T.; Belza, A.; Jensen, M.G.; Ritz, C.; Bitz, C.; Hels, O.; Frandsen, E.; Mela, D.J.; Astrup, A. Acute effects of mustard, horseradish, black pepper and ginger on energy expenditure, appetite, ad libitum energy intake and energy balance in human subjects. Br. J. Nutr. 2012, 109, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Ito, M. Appetite-enhancing effects of curry oil. Biol. Pharm. Bull. 2016, 39, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D.; Cowart, B.J. Dietary assessment of patients with chemosensotyr disorders. J. Acad. Nutr. Diet. 1994, 94, 50–56. [Google Scholar] [CrossRef]
- Haldar, S.; Chia, S.C.; Lee, S.H.; Lim, J.; Leow, M.K.-S.; Chan, E.C.Y.; Henry, C.J. Polyphenol-rich curry made with mixed spices and vegetables benefits glucose homeostasis in Chinese males (polyspice study): A dose–response randomized controlled crossover trial. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Chia, S.C.; Henry, C.J. Polyphenol-rich curry made with mixed spices and vegetables increases postprandial plasma GLP-1 concentration in a dose-dependent manner. Eur. J. Clin. Nutr. 2018, 72, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Caudwell, P.; Finlayson, G.; Webb, D.-L.; Hellström, P.M.; Näslund, E.; Blundell, J.E. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J. Clin. Endocrinol. Metab. 2013, 98, E847–E855. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O.; Wainstein, J.; Boaz, M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids 2012, 77, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Gruendel, S.; Garcia, A.L.; Otto, B.; Mueller, C.; Steiniger, J.; Weickert, M.O.; Speth, M.; Katz, N.; Koebnick, C. Carob pulp preparation rich in insoluble dietary fiber and polyphenols enhances lipid oxidation and lowers postprandial acylated ghrelin in humans. J. Nutr. 2006, 136, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Panickar, K.S. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Mol. Nutr. Food Res. 2013, 57, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Kanellos, P.T.; Gioxari, A.; Karathanos, V.T. Regulation of gip and ghrelin in healthy subjects fed on sun-dried raisins: A pilot study with a crossover trial design. J. Med. Food 2017, 20, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Depoortere, I.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols. Mol. Nutr. Food Res. 2016, 60, 2554–2564. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Magson, L.D.; Blundell, J.E. Hunger and palatability: Tracking ratings of subjective experience before, during and after the consumption of preferred and less preferred food. Appetite 1984, 5, 361–371. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24. [Google Scholar] [CrossRef]
- Chapman, I.M.; Goble, E.A.; Wittert, G.A.; Morley, J.E.; Horowitz, M. Effect of intravenous glucose and euglycemic insulin infusions on short-term appetite and food intake. Am. J. Physiol. 1998, 274, R596–R603. [Google Scholar] [CrossRef] [PubMed]
- Falkén, Y.; Hellström, P.M.; Sanger, G.J.; Dewit, O.; Dukes, G.; Grybäck, P.; Holst, J.J.; Näslund, E. Actions of prolonged ghrelin infusion on gastrointestinal transit and glucose homeostasis in humans. Neurogastroenterol. Motil. 2010, 22, e192–e200. [Google Scholar] [CrossRef] [PubMed]
- Edholm, T.; Degerblad, M.; Grybäck, P.; Hilsted, L.; Holst, J.J.; Jacobsson, H.; Efendic, S.; Schmidt, P.T.; Hellström, P.M. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol. Motil. 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Hlebowicz, J.; Hlebowicz, A.; Lindstedt, S.; Björgell, O.; Höglund, P.; Holst, J.J.; Darwiche, G.; Almér, L.-O. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am. J. Clin. Nutr. 2009, 89, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Zanzer, Y.C.; Plaza, M.; Dougkas, A.; Turner, C.; Björck, I.; Östman, E. Polyphenol-rich spice-based beverages modulated postprandial early glycaemia, appetite and pyy after breakfast challenge in healthy subjects: A randomized, single blind, crossover study. J. Funct. Foods 2017, 35, 574–583. [Google Scholar] [CrossRef]
- Maji Amal, K.; Banerji, P. Phytochemistry and gastrointestinal benefits of the medicinal spice, capsicum annuum l (chilli): A review. J. Complement. Integr. Med. 2016, 13, 97–122. [Google Scholar] [CrossRef] [PubMed]
- Ghawi, S.K.; Rowland, I.; Methven, L. Enhancing consumer liking of low salt tomato soup over repeated exposure by herb and spice seasonings. Appetite 2014, 81, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Knaapila, A.; Laaksonen, O.; Virtanen, M.; Yang, B.; Lagström, H.; Sandell, M. Pleasantness, familiarity, and identification of spice odors are interrelated and enhanced by consumption of herbs and food neophilia. Appetite 2017, 109, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Griffioen-Roose, S.; Mars, M.; Finlayson, G.; Blundell, J.E.; de Graaf, C. Satiation due to equally palatable sweet and savory meals does not differ in normal weight young adults. J. Nutr. 2009, 139, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, N.K.; Hayes, J.E. Behavioral measures of risk tasking, sensation seeking and sensitivity to reward may reflect different motivations for spicy food liking and consumption. Appetite 2016, 103, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, M.R. Palatability and the micro-structure of feeding in humans: The appetizer effect. Appetite 1996, 27, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, M.R. Taste, palatability and the control of appetite. Proc. Nutr. Soc. 1998, 57, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, D.P.; Lakemond, C.M.M.; de Wijk, R.A.; Luning, P.A.; de Graaf, C. Both longer oral sensory exposure to and higher intensity of saltiness decrease ad libitum food intake in healthy normal-weight men. J. Nutr. 2011, 141, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, M.G.; Luning, P.A.; Ruijschop, R.M.A.J.; Lakemond, C.M.M.; Bult, J.H.F.; Gort, G.; van Boekel, M.A.J.S. Aroma exposure time and aroma concentration in relation to satiation. Br. J. Nutr. 2013, 111, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Ruijschop, R.M.A.J.; Boelrijk, A.E.M.; de Graaf, C.; Westerterp-Plantenga, M.S. Retronasal aroma release and satiation: A review. J. Agric. Food Chem. 2009, 57, 9888–9894. [Google Scholar] [CrossRef] [PubMed]

| Measurement | D0C (Mean ± SD)(n = 20) | D1C (Mean ± SD)(n = 17) | D2C (Mean ± SD)(n = 20) | Pairwise Comparison * |
|---|---|---|---|---|
| Total Ghrelin (ΔAUC) | −78,098.98 ± 41,101.98 | −76,549.46 ± 41,482.70 | −78,883.94 ± 40,022.88 | ND |
| ‘Hunger’ (ΔAUC) | −4659.45 ± 3272.36 | −7179.61 ± 3782.34 | −7020.96 ± 3871.20 | D0C vs. D1C (p = 0.017) |
| D0C vs. D2C (p = 0.028) | ||||
| ‘Fullness’ (ΔAUC) | 6393.71 ± 3681.80 | 7764.54 ± 3908.07 | 7850.15 ± 3581.19 | ND |
| ‘Desire to Eat’(ΔAUC) | −4495.00 ± 3194.31 | −7268.09 ± 4053.52 | −7194.88 ± 3849.50 | D0C vs. D1C (p = 0.002) |
| D0C vs. D2C (p = 0.005) | ||||
| ‘Prospective eating’ (ΔAUC) | −4913.78 ± 3401.05 | −6095.76 ± 3612.99 | −5883.42 ± 3506.46 | ND |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haldar, S.; Lim, J.; Chia, S.C.; Ponnalagu, S.; Henry, C.J. Effects of Two Doses of Curry Prepared with Mixed Spices on Postprandial Ghrelin and Subjective Appetite Responses—A Randomized Controlled Crossover Trial. Foods 2018, 7, 47. https://doi.org/10.3390/foods7040047
Haldar S, Lim J, Chia SC, Ponnalagu S, Henry CJ. Effects of Two Doses of Curry Prepared with Mixed Spices on Postprandial Ghrelin and Subjective Appetite Responses—A Randomized Controlled Crossover Trial. Foods. 2018; 7(4):47. https://doi.org/10.3390/foods7040047
Chicago/Turabian StyleHaldar, Sumanto, Joseph Lim, Siok Ching Chia, Shalini Ponnalagu, and Christiani Jeyakumar Henry. 2018. "Effects of Two Doses of Curry Prepared with Mixed Spices on Postprandial Ghrelin and Subjective Appetite Responses—A Randomized Controlled Crossover Trial" Foods 7, no. 4: 47. https://doi.org/10.3390/foods7040047





