Lactic Acid Bacteria from Kefir Increase Cytotoxicity of Natural Killer Cells to Tumor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Preparation of Lactic Acid Bacteria Mixture
2.4. Measurement of NK Cell Activity and Tumor Cytotoxicity
2.5. RT-PCR (Reverse Transcription Polymerase Chain Reaction)
2.6. IFN-γ Measurement
2.7. Statistical Analysis
3. Results and Discussion
3.1. Induction of NK Activity by the Six Lactic Acid Bacteria from Homemade-Kefir
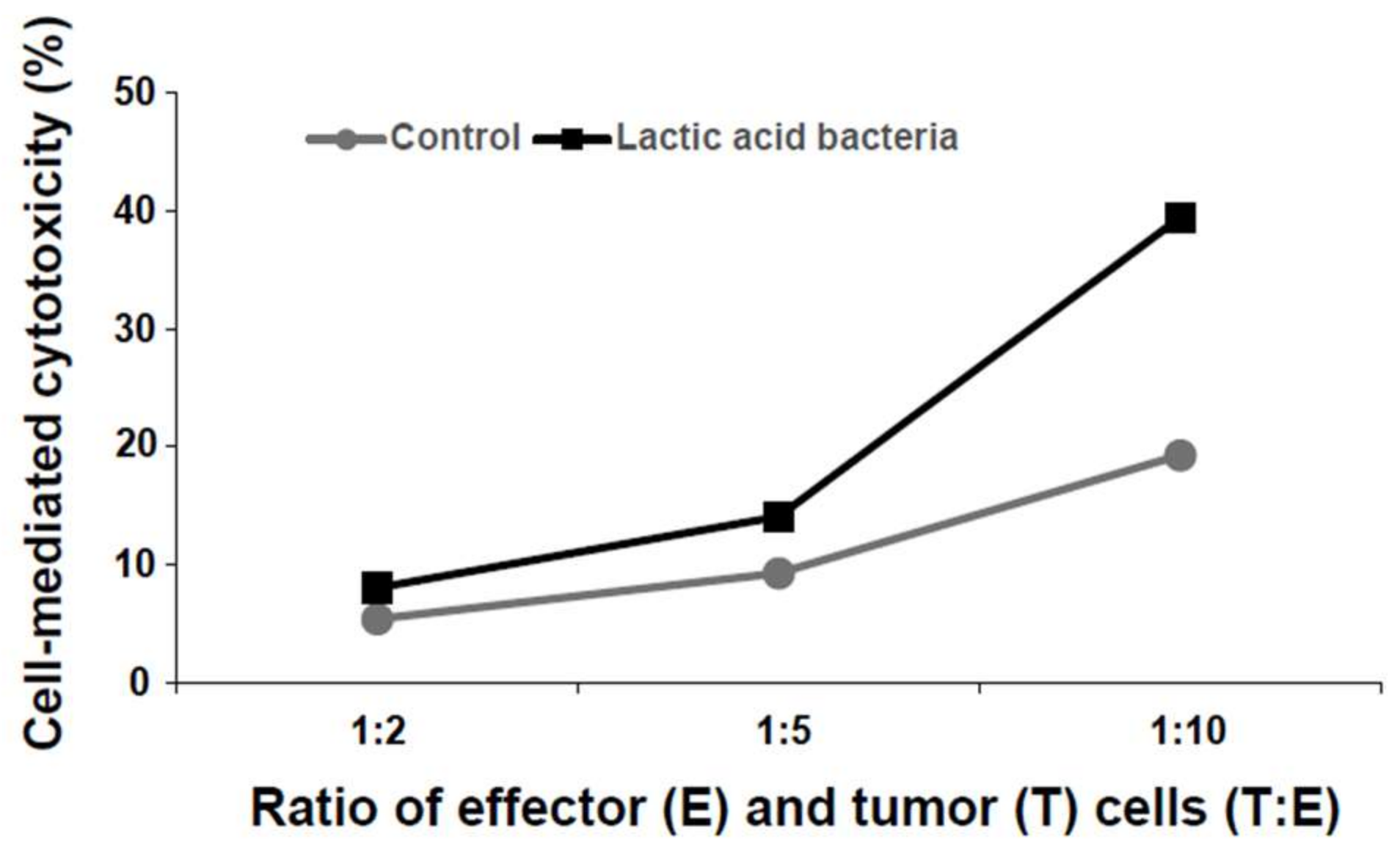
3.2. Induction of Cytotoxicity by the Six Lactic Acid Bacteria Against Human Colorectal Tumor Cells
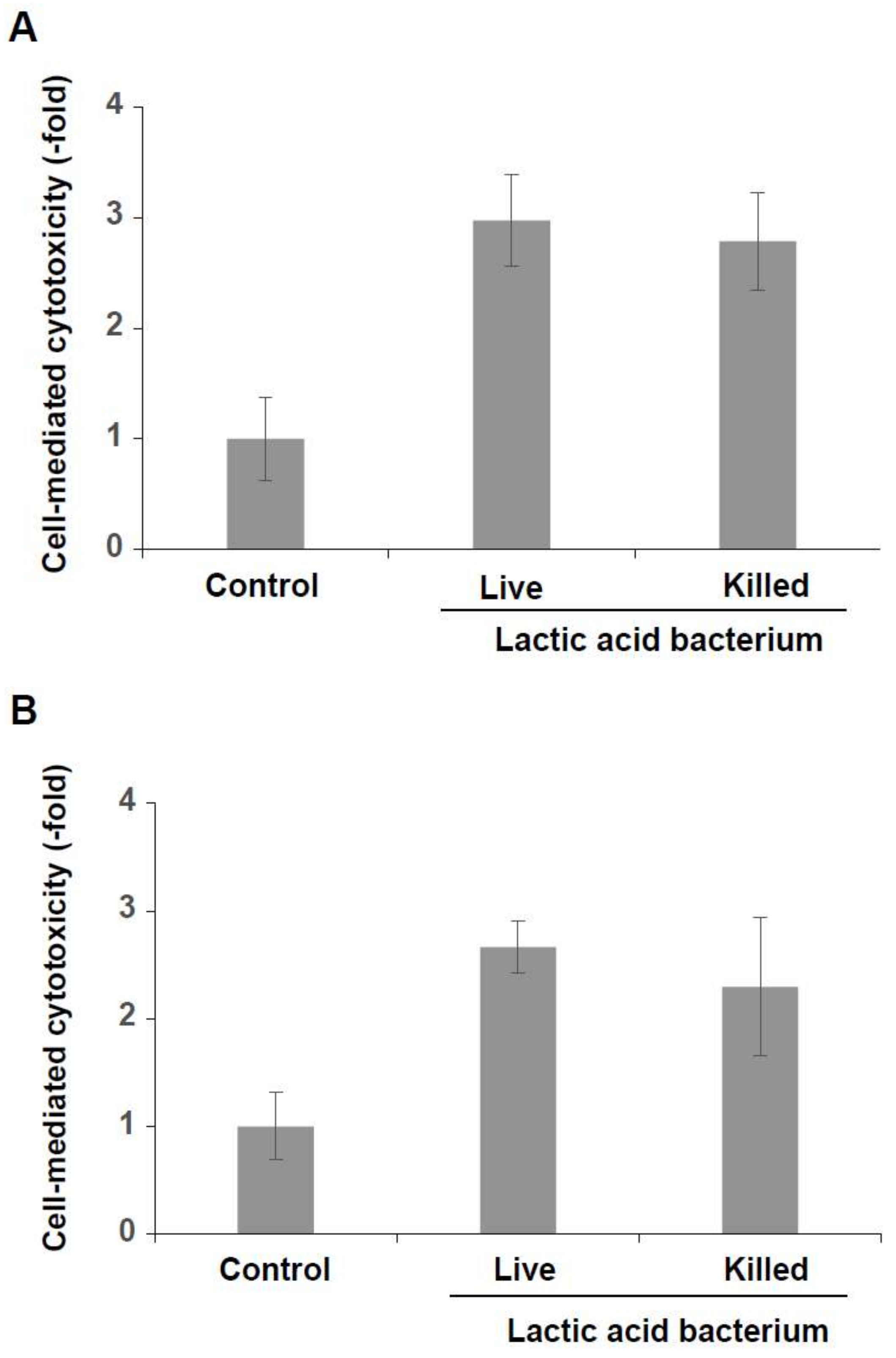
3.3. Induction of Cytotoxicity of KHYG-1 Cells by the Killed Lactic Acid Bacteria Mixture
3.4. Increased Expression and Secretion Levels of IFN-γ in KHYG-1 Cells
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bourrie, B.C.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo, L.; Lopez, E.; Lema, C. Microflora present in kefir grains of the Galician region (North-West of Spain). J. Dairy Res. 1993, 60, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Pintado, M.E.; Da Silva, J.A.L.; Fernandes, P.B.; Malcata, F.X.; Hogg, T.A. Microbiological and rheological studies on Portuguese kefir grains. Int. J. Food Sci. Technol. 1996, 31, 15–26. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 2001, 68, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Simova, E.; Beshkova, D.; Angelov, A.; Hristozova, T.; Frengova, G.; Spasov, Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biotechnol. 2002, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Witthuhn, R.C.; Schoeman, T.; Britz, T.J. Isolation and characterization of the microbial population of different South African kefir grains. Int. J. Dairy Technol. 2004, 57, 33–37. [Google Scholar] [CrossRef]
- Witthuhn, R.; Schoeman, T.; Britz, T. Characterisation of the microbial population at different stages of Kefir production and Kefir grain mass cultivation. Int. Dairy J. 2005, 15, 383–389. [Google Scholar] [CrossRef]
- Yüksekdağ, Z.; Beyatli, Y.; Aslim, B. Determination of some characteristics coccoid forms of lactic acid bacteria isolated from Turkish kefirs with natural probiotic. LWT Food Sci. Technol. 2004, 37, 663–667. [Google Scholar] [CrossRef]
- Mainville, I.; Robert, N.; Lee, B.; Farnworth, E.R. Polyphasic characterization of the lactic acid bacteria in kefir. Syst. Appl. Microbiol. 2006, 29, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Wang, S.-Y.; Chen, M.-J. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 2008, 25, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Osimani, A.; Milanović, V.; Aquilanti, L.; De Filippis, F.; Stellato, G.; Di Mauro, S.; Turchetti, B.; Buzzini, P.; Ercolini, D.; et al. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 2015, 49, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zanirati, D.F.; Abatemarco, M.; de Cicco Sandes, S.H.; Nicoli, J.R.; Nunes, Á.C.; Neumann, E. Selection of lactic acid bacteria from Brazilian kefir grains for potential use as starter or probiotic cultures. Anaerobe 2015, 32, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Korsak, N.; Taminiau, B.; Leclercq, M.; Nezer, C.; Crevecoeur, S.; Ferauche, C.; Detry, E.; Delcenserie, V.; Daube, G. Short communication: Evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J. Dairy Sci. 2015, 98, 3684–3689. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; San Mauro, M.; Sanchez, A.; Torres, J.M.; Marquina, D. The antimicrobial properties of different strains of Lactobacillus spp. isolated from kefir. Syst. Appl. Microbiol. 2003, 26, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Pedrozo Miguel, M.G.; Cardoso, P.G.; de Assis Lago, L.; Schwan, R.F. Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods. Food Res. Int. 2010, 43, 1523–1528. [Google Scholar] [CrossRef]
- Nalbantoglu, U.; Cakar, A.; Dogan, H.; Abaci, N.; Ustek, D.; Sayood, K.; Can, H. Metagenomic analysis of the microbial community in kefir grains. Food Microbiol. 2014, 41, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Chouraqui, J.P.; Van Egroo, L.D.; Fichot, M.C. Acidified milk formula supplemented with Bifidobacterium lactis: Impact on infant diarrhea in residential care settings. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Gaón, D.; García, H.; Winter, L.; Rodríguez, N.; Quintás, R.; González, S.N.; Oliver, G. Effect of Lactobacillus strains and Saccharomyces boulardii on persistent diarrhea in children. Medicina (Buenos Aires) 2003, 63, 293–298. [Google Scholar]
- Pohjavuori, E.; Viljanen, M.; Korpela, R.; Kuitunen, M.; Tiittanen, M.; Vaarala, O.; Savilahti, E. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow’s milk allergy. J. Allergy Clin. Immunol. 2004, 114, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Bourlioux, P.; Koletzko, B.; Guarner, F.; Braesco, V. The intestine and its microflora are partners for the protection of the host: Report on the Danone Symposium “The Intelligent Intestine,” held in Paris, June 14, 2002. Am. J. Clin. Nutr. 2003, 78, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Azcárate-Peril, M.A.; Sikes, M.; Bruno-Bárcena, J.M. The intestinal microbiota, gastrointestinal environment and colorectal cancer: A putative role for probiotics in prevention of colorectal cancer? Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G401–G424. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen, S.; de LeBlanc, A.M.; Miyoshi, A.; Rocha, C.S.; Azevedo, V.; LeBlanc, J.G. Potential application of probiotics in the prevention and treatment of inflammatory bowel diseases. Ulcers 2011, 2011, 841651. [Google Scholar] [CrossRef]
- Rafter, J.J. The role of lactic acid bacteria in colon cancer prevention. Scand. J. Gastroenterol. 1995, 30, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K.; Rafter, J. The role of lactic acid bacteria in colon cancer prevention: Mechanistic considerations. Antonie Van Leeuwenhoek 1999, 76, 391–394. [Google Scholar] [PubMed]
- Nagao, F.; Nakayama, M.; Muto, T.; Okumura, K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci. Biotechnol. Biochem. 2000, 64, 2706–2708. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Tuohy, K.M.; Thomas, L.V.; Yaqoob, P. Selective effects of Lactobacillus casei Shirota on T cell activation, natural killer cell activity and cytokine production. Clin. Exp. Immunol. 2010, 161, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Kobayashi, M.; Okamoto, T. Rapid PCR-Based Method Which Can Determine Both Phenotype and Genotype of Lactococcus lactis Subspecies. Appl. Environ. Microbiol. 2002, 68, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Beimfohr, C.; Ludwig, W.; Schleifer, K.H. Rapid Genotypic Differentiation of Lactococcus lactis Subspecies and Biovar. Syst. Appl. Microbiol. 1997, 20, 216–221. [Google Scholar] [CrossRef]
- Feutry, F.; Torre, P.; Arana, I.; Garcia, S.; Desmasures, N.; Casalta, E. Lactococcus lactis strains from raw ewe’s milk samples from the PDO Ossau-Iraty cheese area: Levels, genotypic and technological diversity. Dairy Sci. Technol. 2012, 92, 655–670. [Google Scholar] [CrossRef]
- Kahala, M.; Maki, M.; Lehtovaara, A.; Tapanainen, J.M.; Katiska, R.; Juuruskorpi, M.; Juhola, J.; Joutsjoki, V. Characterization of starter lactic acid bacteria from the Finnish fermented milk product viili. J. Appl. Microbiol. 2008, 105, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.J.H.; Timmins, M.J. Differentiation of Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus by polymerase chain reaction. Lett. Appl. Microbiol. 1999, 29, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, X.; Covasa, M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. 2014, 20, 7878–7886. [Google Scholar] [CrossRef] [PubMed]
- De Montijo-Prieto, S.; Moreno, E.; Bergillos-Meca, T.; Lasserrot, A.; Ruiz-López, M.D.; Ruiz-Bravo, A.; Jiménez-Valera, M.A. Lactobacillus plantarum strain isolated from kefir protects against intestinal infection with Yersinia enterocolitica O9 and modulates immunity in mice. Res. Microbiol. 2015, 166, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.E.; Keating, N.; Belz, G.T. Natural killer cells and anti-tumor immunity. Mol. Immunol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Kang, H.; Cho, H. Natural killer cells and tumor metastasis. Arch. Pharm. Res. 2017, 40, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Tokatlı, M.; Gülgör, G.; Bağder Elmacı, S.; Arslankoz İşleyen, N.; Özçelik, F. In Vitro Properties of Potential Probiotic Indigenous Lactic Acid Bacteria Originating from Traditional Pickles. BioMed Res. Int. 2015, 2015, 315819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Tan, Z.; Li, Z.; Jiao, Z.; Huang, Q. Screening of Probiotic Activities of Lactobacilli Strains Isolated from Traditional Tibetan Qula, A Raw Yak Milk Cheese. Asian-Australas. J. Anim. Sci. 2016, 29, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Old, L.J.; Schreiber, R.D. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002, 13, 95–109. [Google Scholar]
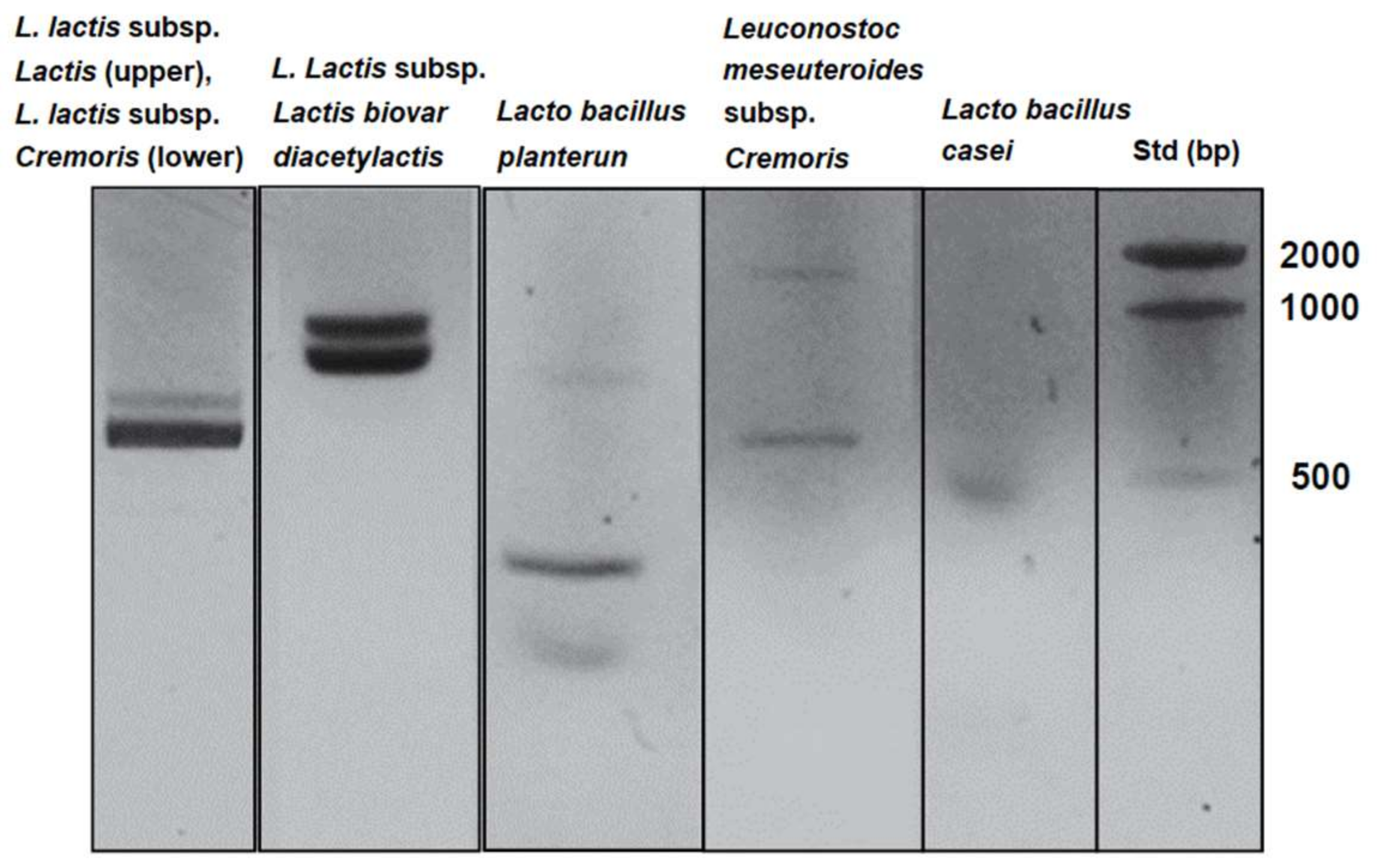


| Strain | Primer Sequence | PCR Condition | Ref. | |
|---|---|---|---|---|
| L. lactis subsp. Lactis | sense | 5′-CGTTATGGATTTGATGGATATAAAGC-3′ | 94 °C 9 min, 94 °C 30 s, 50 °C 30 s, 72 °C 60 s × 45 cycles, 72 °C 7 min | [27] |
| antisense | 5′-ACTCTTCTTAAGAACAAGTTTAACAGC-3′ | |||
| L. lactis subsp. Cremoris | sense | 5′-CGTTATGGATTTGATGGATATAAAGC-3′ | 94 °C 9 min, 94 °C 30 s, 50 °C 30 s, 72 °C 60 s × 45 cycles, 72 °C 7 min | |
| antisense | 5′-ACTCTTCTTAAGAACAAGTTTAACAGC-3′ | |||
| L. lactis subsp. lactis biovar diacetylactis | sense | 5′-CTTCGTTATGATTTTACA-3′ | 94 °C 30 s, 94 °C 30 s, 46 °C 60 s, 72 °C 60 s × 30 cycles, 72 °C 2 min | [28,29] |
| antisense | 5′-AATATCAACAATTCCATG-3′ | |||
| Leuconostoc mesenteroides subsp. Cremoris (Genus-specific primers for Leuconostoc) | sense | 5′-AACTTAGTGTCGCATGAC-3′ | 94 °C 5 min, 94 °C 60 s, 60 °C 60 s, 72 °C 60 s × 30 cycles, 72 °C 10 min | [30] |
| antisense | 5′-AGTCGAGTTACAGACTACAA-3′ | |||
| Lactobacillus plantarum | sense | 5′-CCGTTTATGCGGAACACCTA-3′ | 94 °C 3 min, 94 °C 30 s, 54 °C 10 s, 72 °C 30 s × 30 cycles, 72 °C 5 min | [31] |
| antisense | 5′-TCGGGATTACCAAACATCAC-3′ | |||
| Lactobacillus casei | sense | 5-TGCACTGAGATTCGACTTAA-3 | 94 °C 3 min, 94 °C 45 s, 45 °C 45 s, 72 °C 60 s × 30 cycles, 72 °C 5 min | [32] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamane, T.; Sakamoto, T.; Nakagaki, T.; Nakano, Y. Lactic Acid Bacteria from Kefir Increase Cytotoxicity of Natural Killer Cells to Tumor Cells. Foods 2018, 7, 48. https://doi.org/10.3390/foods7040048
Yamane T, Sakamoto T, Nakagaki T, Nakano Y. Lactic Acid Bacteria from Kefir Increase Cytotoxicity of Natural Killer Cells to Tumor Cells. Foods. 2018; 7(4):48. https://doi.org/10.3390/foods7040048
Chicago/Turabian StyleYamane, Takuya, Tatsuji Sakamoto, Takenori Nakagaki, and Yoshihisa Nakano. 2018. "Lactic Acid Bacteria from Kefir Increase Cytotoxicity of Natural Killer Cells to Tumor Cells" Foods 7, no. 4: 48. https://doi.org/10.3390/foods7040048




