Refrigerated Shelf Life of a Coconut Water-Oatmeal Mix and the Viability of Lactobacillus Plantarum Lp 115-400B
Abstract
:1. Introduction
2. Experimental Section
2.1. Probiotic Culture
2.2. Substrate and Substrate Preparation
2.3. Fermentation
2.4. Shelf-life Analysis
2.5. pH and Acidity Analysis
2.6. Rheological Measurements
2.7. Statistical Analysis
3. Results and Discussion
3.1. Viability Analysis during the Refrigeration Storage of Probiotic Fermented Oatmeal
| Time (day) | Log CFU/g (P) | Log CFU/g (PP) |
|---|---|---|
| 0 | 7.06 ± 0.27A a | 6.99 ± 0.27A |
| 7 | 9.12 ± 0.01B | 9.01 ± 0.11B |
| 14 | 8.89 ± 0.02B | 8.75 ± 0.04B |
| 21 | 8.37 ± 0.22C | 7.97 ± 0.15C |
| 28 | 8.01 ± 0.15C | 7.46 ± 0.03D |
| 35 | 7.75 ± 0.15D | 7.05 ± 0.08A |
| 42 | 7.54 ± 0.10D | 6.66 ± 0.07E |
| 49 | 7.23 ± 0.02A | 6.41 ± 0.06E |
3.2. pH and Acidity Changes of Coconut Water-Oatmeal Matrix
| Time (day) | pH of P | pH of PP | pH of C |
|---|---|---|---|
| 0 | 5.78 ± 0.00A a | 5.75 ± 0.03A | 5.79 ± 0.01A |
| 7 | 5.23 ± 0.07C | 5.08 ± 0.07B | 6.22 ± 0.03B |
| 14 | 5.18 ± 0.08C | 5.04 ± 0.17B | 6.23 ± 0.03B |
| 21 | 5.20 ± 0.13C | 5.07 ± 0.20B | 6.24 ± 0.02B |
| 28 | 5.21 ± 0.16C | 5.08 ± 0.23B | 6.25 ± 0.02B |
| 35 | 5.22 ± 0.14C | 5.02 ± 0.27B | 6.17 ± 0.05B |
| 42 | 5.45 ± 0.11D | 5.15 ± 0.36B | 6.21 ± 0.02B |
| 49 | 5.43 ± 0.11D | 5.12 ± 0.33B | 6.24 ± 0.01B |
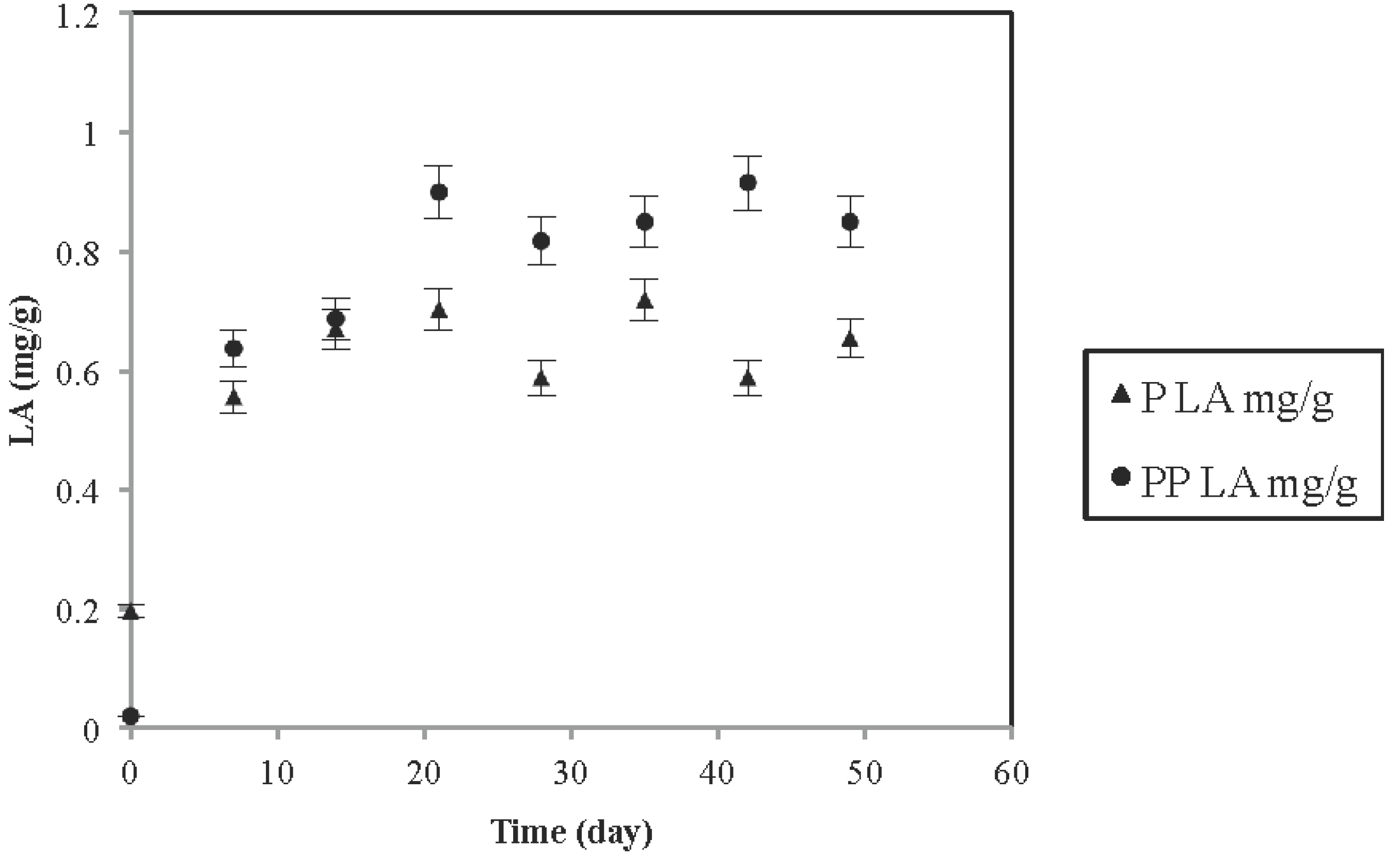
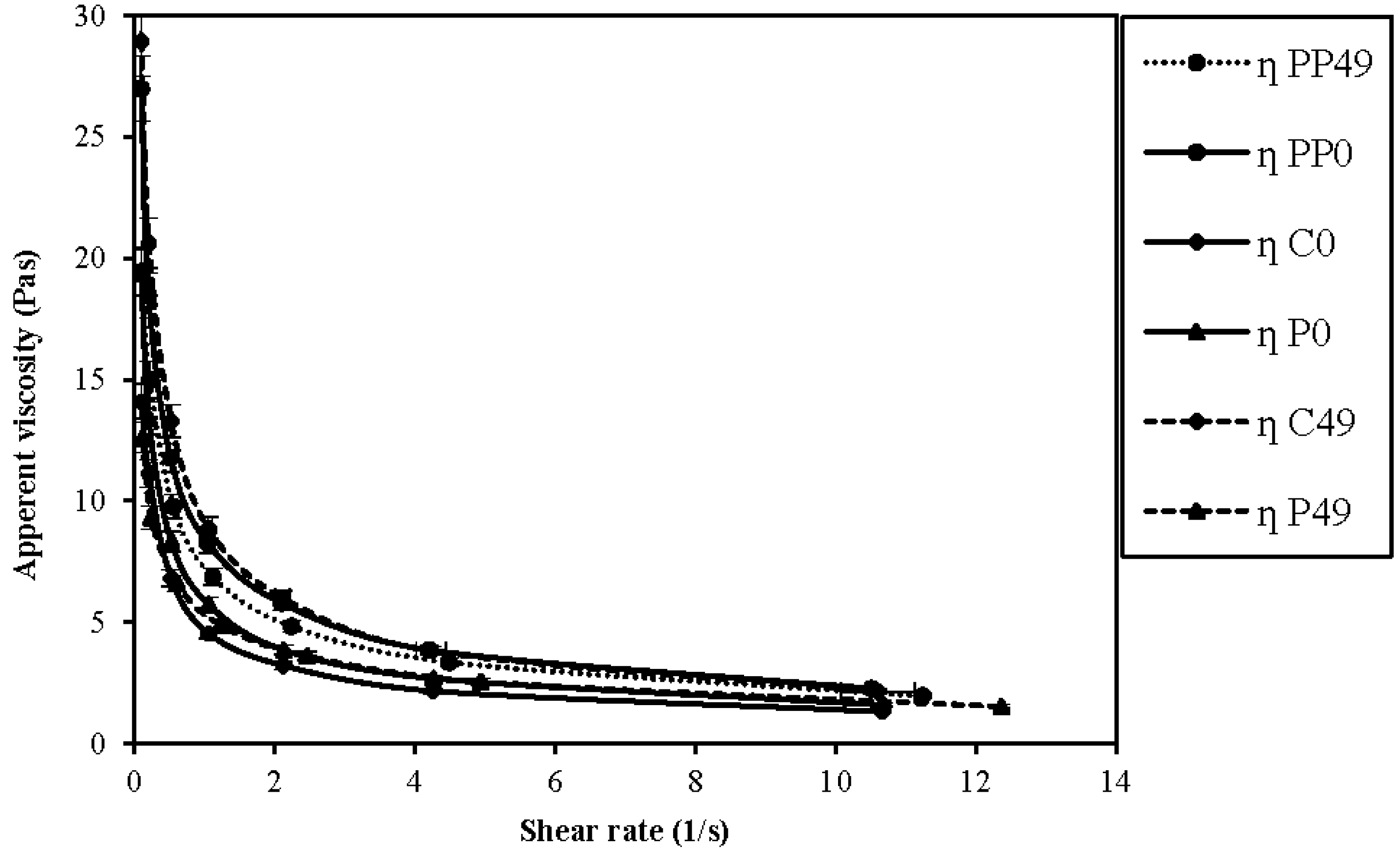
3.3. Rheological Parameters: Viscosity Analysis
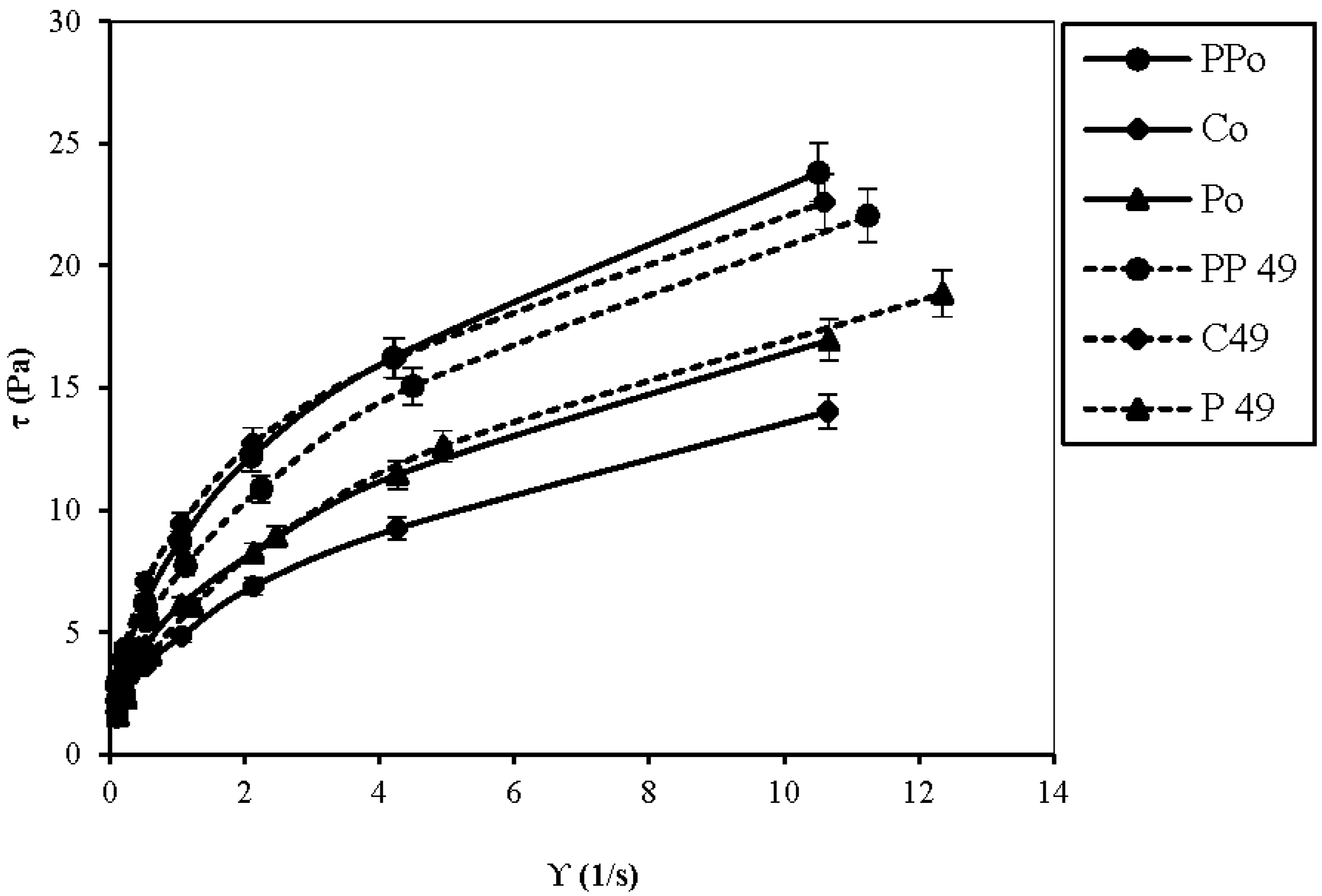
| Sample | Flow Behavior Index (n) on Day 0 | Flow Behavior Index (n) on Day 49 |
|---|---|---|
| C | 0.47 ± 0.01A a | 0.46 ± 0.04A |
| P | 0.46 ± 0.02A | 0.50 ± 0.06A |
| PP | 0.46 ± 0.01A | 0.51 ± 0.03A |
4. Conclusions
Author Contributions
Conflicts of Interest
References
- FAO. Probiotics in food: Health and nutritional properties and guidelines for evaluation; FAO food and nutrition paper 85; FAO: Rome, Italy, 2006. [Google Scholar]
- Lourens-Hattingh, A.; Viljoen, B.C. Yogurt as probiotic carrier food. Int. Dairy J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Shah, N.P. Probiotics—From Metchnikoff to bioactives. Int. Dairy J. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fonden, R.; Matto, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Leschied, D.W. Probiotics and mucosal immunity: Strain specific effects on Th1/Th2 cell modulation. Int. J. Naturop. Med. 2009, 4, 18–22. [Google Scholar]
- Walker, W.A.; Goulet, O.; Morelli, L.; Antoine, J. Progress in the science of probiotics: From cellular microbiology and applied immunology to clinical nutrition. Eur. J. Nutr. 2006, 45, 1–18. [Google Scholar] [CrossRef]
- Baroukoub, A.; Mehdi, R.Z.; Beglarian, R.; Hassan, J.; Zahra, S.; Mohommad, M.S. Effects of probiotic yoghurt consumption on the serum cholesterol levels hypercholestromic cases in Shiraz Southern Iran. Sci. Res. Essays 2010, 5, 2206–2209. [Google Scholar]
- Zhang, Y.; Li, S.; Gan, R.; Zhou, T.; Xu, D.; Li, H. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Finelly, C. Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Futur. Microbiol. 2015, 10, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Gueimonde, M.; Ouwehand, A.C.; Reinikainen, J.P.; Salminen, S.J. Comparison of four methods to enumerate probiotic bifidobacteria in a fermented food product. Food Microbiol. 2006, 23, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Mortazavian, A.M.; Mohammadifar, M.A.; Koushki, M.R.; Mohammadi, A.; Mohammadi, R. Combined effects of dry matter content, incubation temperature and final pH of fermentation on biochemical and microbiological characteristics of probiotic fermented milk. Afr. J. Microbiol. Res. 2010, 4, 1265–1274. [Google Scholar]
- Charalampopoulos, D.; Pandiella, S.S.; Webb, C. Growth studies of potentially probiotic lactic acid bacteria in cereal-based substrates. J. Appl. Microbiol. 2002, 92, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E. Probiotics. Food Technol. 1999, 53, 67–77. [Google Scholar]
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a new oat-based probiotic drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ranadheer, R.D.S.C.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Martensson, O.; Andersson, C.; Andersson, K.; Oste, R.; Holst, O. Formulation of an oat based product and its comparison with yoghurt. J. Sci. Food Agric. 2001, 81, 1314–1321. [Google Scholar] [CrossRef]
- Kontula, P.; von Wright, A.; Mattila-Sandholm, T. Oat bran β-gluco- and xylo-oligosaccharides as fermentative substrates for lactic acid bacteria. Int. J. Food Microbiol. 1998, 45, 163–169. [Google Scholar] [CrossRef]
- Karmally, W.; Montez, M.G.; Martinez, W.; Branstetter, A.; Ramakrishnan, R.; Holleran, S.F.; Haffner, S.M.; Ginsberg, H.N. Cholesterol-lowering benefits of oat-containing cereal in Hispanic Americans. J. Am. Diet Assoc. 2005, 105, 967–970. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Mitschka, P. Simple conversion of Brookfield R.V.T. readings into viscosity functions. Rheol. Acta. 1982, 21, 207–209. [Google Scholar] [CrossRef]
- Derzelle, S.; Hallet, B.; Ferain, T.; Delcour, J.; Hols, P. Improved adaptation of cold-shock, stationary-phase, and freezing stresses in Lactobacillus plantarum overproducing cold-shock proteins. Appl. Environ. Microbiol. 2003, 69, 4285–4290. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, R.; Iliev, I.; Haertle, T.; Chobert, J.; Ivanova, I.; Danova, S. Technological properties of candidate probiotic Lactobacillus plantarum strains. Int. Dairy J. 2009, 19, 696–702. [Google Scholar] [CrossRef]
- Kalui, C.M.; Mathara, J.M.; Kutima, P.M.; Kiiyukia, C.; Wongo, L.E. Functional characteristics of Lactobacillus plantarum and Lactobacillus rhamnosus from ikii, a Kenyan traditional fermented maize porridge. Afr. J. Biotechnol. 2009, 8, 4363–4373. [Google Scholar]
- Takemura, N.; Ozawa, K.; Kimura, N.; Watanabe, J.; Sonoyama, K. Inulin-type fructans stimulated the growth of exogenously administered Lactobacillus plantarum No. 14 in the mouse gastrointestinal tract. Biosci. Biotechnol. Biochem. 2010, 74, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Molin, G. Probiotics in foods not containing milk or milk constitutes, with special references to Lactobacillus plantarum 299v. Am. J. Nutr. 2001, 73 (Suppl. S2), 380S–385S. [Google Scholar]
- Champagne, C.P.; Gardner, N.J. Challenges in the addition of probiotic cultures to foods. Crit. Rev. Food Sci. 2005, 45, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Lelong, B.; Raimbault, M. Influence of pH and initial lactate concentration on the growth of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 1991, 36, 96–99. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Process optimization for the development of a functional beverage based on lactic acid fermentation of oats. Biochem. Eng. J. 2010, 52, 199–204. [Google Scholar] [CrossRef]
- Tallon, R.; Bressollier, P.; Urdaci, M.C. Isolation and characterization of two exo-polysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 2003, 154, 705–712. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Vanderveken, F.; van de Ven, S.; Degeest, B. Production by and isolation of exo-polysaccharides from Streptococcus thermophilus grown in a milk medium and evidence for their growth-associated biosynthesis. J. Appl. Microbiol. 1998, 84, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmasena, M.; Barron, F.; Fraser, A.; Jiang, X. Refrigerated Shelf Life of a Coconut Water-Oatmeal Mix and the Viability of Lactobacillus Plantarum Lp 115-400B. Foods 2015, 4, 328-337. https://doi.org/10.3390/foods4030328
Dharmasena M, Barron F, Fraser A, Jiang X. Refrigerated Shelf Life of a Coconut Water-Oatmeal Mix and the Viability of Lactobacillus Plantarum Lp 115-400B. Foods. 2015; 4(3):328-337. https://doi.org/10.3390/foods4030328
Chicago/Turabian StyleDharmasena, Muthu, Felix Barron, Angela Fraser, and Xiuping Jiang. 2015. "Refrigerated Shelf Life of a Coconut Water-Oatmeal Mix and the Viability of Lactobacillus Plantarum Lp 115-400B" Foods 4, no. 3: 328-337. https://doi.org/10.3390/foods4030328





