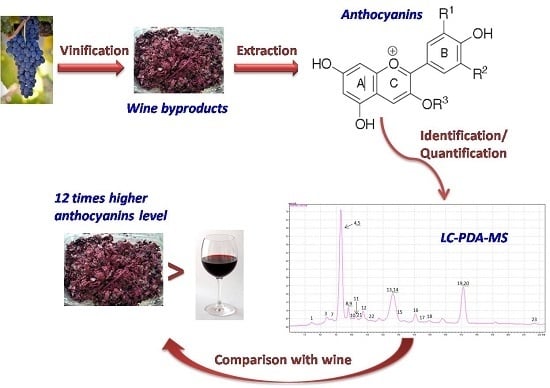
A Sensitive LC-MS Method for Anthocyanins and Comparison of Byproducts and Equivalent Wine Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents and Solutions
2.3. Extraction Procedure
3. Results and Discussion
3.1. Optimization of the Extraction Procedure
3.1.1. Extraction Solution Selection
3.1.2. Dried Matter–to–Solvent Volume Ratio Optimization
3.1.3. Optimization of Water Bath Extraction Duration
3.1.4. Consecutive Extraction Rounds Optimization
3.2. Mobile Phase Selection
3.3. Figures of Merit of the Proposed Method
3.4. Identification and Quantification of Anthocyanins in Wine Variety (Syrah) and Its Byproducts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Mv | Malvidin |
| LC | liquid chromatography |
| PDA | photodiode array |
| MS | mass spectrometry |
References
- Bueno, J.; Ramos-Escudero, F.; Saez-Plaza, P.; Munoz, A.; Navas, M.J.; Asuero, A. Analysis and antioxidant capacity of anthocyanin pigments. Part I: General considerations concerning polyphenols and flavonoids. Crit. Rev. Anal. Chem. 2012, 42, 102–105. [Google Scholar] [CrossRef]
- Bueno, J.; Saez-Plaza, P.; Ramos-Escudero, F.; Jimenez, A.; Fett, R.; Asuero, A. Analysis and antioxidant capacity of anthocyanin pigments. Part II: Chemical structure, color, and intake of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Navas, M.; Jimenez, A.; Bueno, J.; Saez-Plaza, P.; Asuero, A. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part III: An Introduction to Sample Preparation and Extraction. Crit. Rev. Anal. Chem. 2012, 42, 284–312. [Google Scholar] [CrossRef]
- Navas, M.; Jimenez, A.; Bueno, J.; Saez-Plaza, P.; Asuero, A. Analysis and antioxidant capacity of anthocyanin pigments. Part IV: Extraction of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 313–342. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V. A critical review of methods of characterization of polyphenol compounds in fruit and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Cote, J.; Caillet, S.; Doyon, G.; Sylvain, J.; Lacroix, M. Analyzing cranberry bioactive compounds. Crit. Rev. Food Sci. Nutr. 2010, 50, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Unger, M. Capillary electrophoresis of natural products: current applications and recent advances. Planta Med. 2009, 75, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.; Wu, Q.; Simon, J. Recent advances in anthocyanin analysis and characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Cacae, J.; Kay, C. Methods of analysis for anthocyanins in plant and biological fluids. J. AOAC Int. 2004, 87, 129–145. [Google Scholar] [PubMed]
- Kong, J.; Chia, L.; Goh, N.; Chia, T.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Fernandez, O.; Martinez, O.; Hernandez, Z.; Guadalupe, Z.; Ayestaran, B. Effect of the presence of lysated lees on polysaccharides, color and main phenolic compounds of red wine during barrel ageing. Food Res. Int. 2011, 44, 84–91. [Google Scholar] [CrossRef]
- Mazauric, J.; Salmon, J. Interactions between yeast lees and wine polyphenols during simulation of wine aging. II. Analysis of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, C.; Cao, G.; Ou, B.; Prior, R. Anthocyanin and proanthocyanidin content in selected white and red wines. Oxygen radical absorbance capacity comparison with nontraditional wines obtained from highbush blueberry. J. Agric. Food Chem. 2003, 51, 4889–4896. [Google Scholar] [CrossRef] [PubMed]
- Lapornik, B.; Prosek, M.; Wondra, A. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 7, 214–222. [Google Scholar] [CrossRef]
- Revilla, E.; Ryan, J.; Ortega, G. Comparison of several procedures used for the extraction of anthocyanins from red grapes. J. Agric. Food Chem. 1998, 46, 4592–4597. [Google Scholar] [CrossRef]
- Pascual-Teresa, S.; Santos, B.C.; Rivas-Gonzalo, C. LC-MS analysis of anthocyanins from purple corn cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- Arribas, M.; Gomez, C.; Alvarez, P. Evolution of red wine anthocyanins during malolactic fermentation, postfermentative treatments and ageing with lees. Food Chem. 2008, 109, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Garcia, A.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Ju, Z.; Howard, L. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Revilla, I.; Perez, M.S.; Gonzalez, S.M.; Beltran, S. Identification of anthocyanin derivatives in grape skin extracts and red wines by liquid chromatography with diode array and mass spectrometric detection. J. Chromatogr. A 1999, 847, 83–90. [Google Scholar] [CrossRef]
- Hartmanova, L.; Ranc, V.; Papouskova, B.; Bednar, P.; Havlicek, V.; Lemr, K. Fast profiling of anthocyanins in wine by desorption nano-electrospray ionization mass spectrometry. J. Chromatogr. A 2010, 1217, 4223–4228. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, Q.; Cui, X.; Duan, C. Optimization on anthocyanins extraction from wine grape skins using orthogonal test design. Food Sci. Biotechnol. 2010, 19, 1047–1053. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, B.; Fan, P.; Yang, C.; Duan, W.; Zheng, X.; Liu, C.; Li, S. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chem. 2008, 111, 837–844. [Google Scholar] [CrossRef]
- Ling, Y.; Ren, C.; Mallery, S.; Ugalde, C.; Pei, P.; Saradhi, U.; Stoner, G.; Chan, K.; Liu, Z. A rapid and sensitive LC-MS/MS method for quantification of four anthocyanins and its application in a clinical pharmacology study of a bioadhesive black raspberry gel. J. Chromatogr. B 2009, 877, 4027–4034. [Google Scholar] [CrossRef] [PubMed]
- Duymus, G.; Goger, F.; Baser, C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.; Cuevas, E.; Winterhalter, P.; Garcia, M.; Troncoso, A. Anthocyanin composition in Cabernet Sauvignon red wine vinegar obtained by submerged acetification. Food Res. Int. 2010, 43, 1577–1584. [Google Scholar] [CrossRef]
- Papadoyannis, I.; Samanidou, V.; Antoniou, C. Gradient RP-HPLC Determination of Free Phenolic Acids in Wines and Wine Vinegar Samples after SPE, with Photodiode Array Identification. J. Liq. Chromatogr. Relat. Tech. 2001, 24, 2161–2176. [Google Scholar]
- Themelis, D.; Tzanavaras, P.; Anthemidis, A.; Stratis, J. Direct, selective flow injection spectrophotometric determination of calcium in wines using methylthymol blue and an on-line cascade dilution system. Anal. Chim. Acta 1999, 402, 259–266. [Google Scholar] [CrossRef]
- Tzanavaras, P.D.; Thiakouli, E.; Themelis, D. Hybrid sequential injection–flow injection manifold for the spectrophotometric determination of total sulfite in wines using o-phthalaldehyde and gas-diffusion. Talanta 2009, 77, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ariza, L.; Garcia-Barrera, T.; Lorenzo, F. Dynamic headspace coupled to perevaporation for the analysis of anisoles in wine by gas chromatography-ion-trap tandem mass spectrometry. J. Chromatogr. A 2004, 1056, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ariza, L.; Garcia-Barrera, T.; Lorenzo, F.; Beltran, R. Use of multiple headspace solid-phase microextraction and pervaporation for the determination of off-flavours in wine. J. Chromatogr. A 2006, 1112, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Metivier, R.; Francis, F.; Clydesdale, F. Solvent extraction of anthocyanins from wine pomace. J. Food Sci. 1980, 45, 1099–1100. [Google Scholar] [CrossRef]
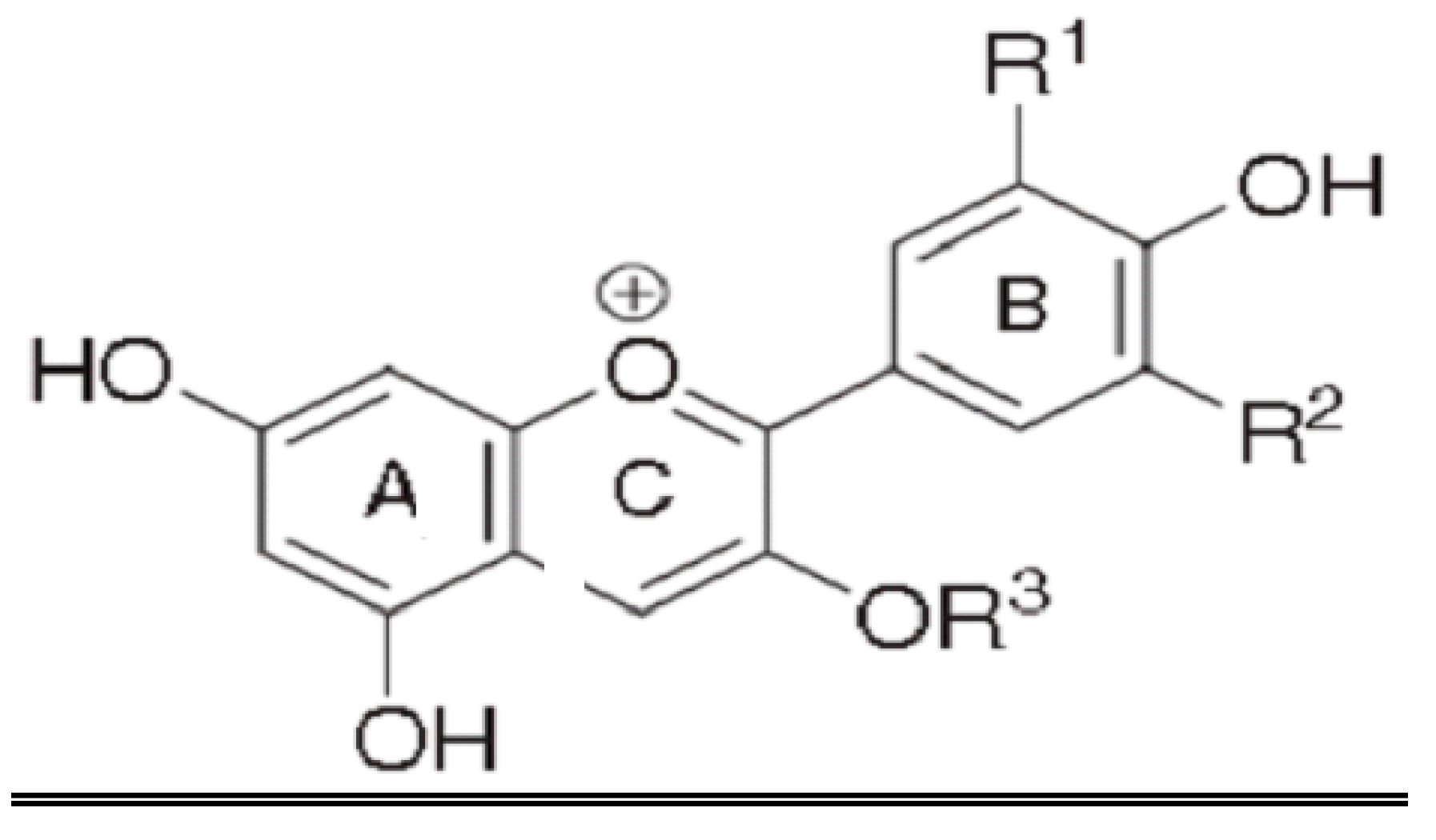



| Name | Abrev. | R1 | R2 | R3 |
|---|---|---|---|---|
| Delphinidin | Dp | OH | OH | H |
| Delphinidin-3-O-glucoside | Dp-3-O-glu | OH | OH | Glc |
| Petunidin | Pt | OH | OCH3 | H |
| Petunidin-3-O-glucoside | Pt-3-O-glu | OH | OCH3 | Glc |
| Malvidin | Mv | OCH3 | OCH3 | H |
| Malvidin-3-O-glucoside | Mv-3-O-glu | OCH3 | OCH3 | Glc |
| Cyanidin | Cn | OH | H | H |
| Cyanidin-3-O-glucoside | Cn-3-O-glu | OH | H | Glc |
| Peonidin | Pn | OCH3 | H | H |
| Peonidin-3-O-glucoside | Pn-3-O-glu | OCH3 | H | Glc |
| Pelargonidin | Pg | H | H | H |
| Pelargonidin-3-O-glucoside | Pg-3-O-glu | H | H | Glc |
| Parameter | Value |
|---|---|
| Ionization process | ESI |
| Ionization mode | Positive |
| Secondary electron multiplier detector voltage | 1.8 kV |
| Capillary voltage | 4.0 kV |
| Mass scanning range | 200 m/z–1200 m/z |
| Solvent A (volume/volume) | Water/Formic Acid (99/1) |
| Solvent B (volume/volume) | Acetonitrile/Formic Acid (99/1) |
| Mobile phase flow-rate | 0.5 mL/min |
| Injection volume | 20 μL |
| Chromatographic Column | Dionex, RP-C18, 150 mm × 4.6 mm × 5 μm |
| Elution program type | Gradient |
| Dry Matter Mass (g) | Solvent Volume (mL) | Dry Matter Mass (g)/Solvent Volume (mL) | mg TAC */100 g sample ** |
|---|---|---|---|
| 0.5 | 5.0 | 1/10 | 99.9 ± 10.1 |
| 0.5 | 10.0 | 1/20 | 117 ± 10.9 |
| 0.5 | 15.0 | 1/30 | 116 ± 10.8 |
| 0.5 | 20.0 | 1/40 | 119 ± 11.3 |
| Duration Time (h) in Water Bath | mg TAC */100 g Sample ** |
|---|---|
| 1 | 93.4 ± 9.41 |
| 2 | 129 ± 11.2 |
| 3 | 128 ± 11.1 |
| 4 | 130 ± 11.6 |
| Successive Extraction Number | mg TAC */100 g Sample ** |
|---|---|
| 1 | 130 ± 14 |
| 2 | 27.6 ± 3.3 |
| 3 | 2.92 ± 0.33 |
| 4 | 0.08 ± 0.02 |
| Slope, S (Peak Area/(mg·L−1)) | Correlation Coefficient, r | Instrumental RSD * | LOD (ng·mL−1) | Dynamic Range (mg·L−1) |
|---|---|---|---|---|
| 1.34 × 105 | 0.9996 | 5.8% | 9.0 | 0.03–10.0 |
| Samples | Concentration in Wine Byproducts (mg 1000 g−1 of DW) * | Concentration in Wine (Syrah) (mg·L−1 of Wine) * | Ratio Between Samples | |
|---|---|---|---|---|
| Anthocyanins Group | ||||
| Simple glucosides | 4367 | 457.7 | 9.54 | |
| Acetyl glucosides | 5224 | 421.3 | 12.40 | |
| Cinnamoyl glucosides | 2779 | 152.7 | 18.20 | |
| Pyroanthocyanins | 73.3 | 12.6 | 5.82 | |
| Total anthocyanins | 12,443.3 | 1044.3 | 11.92 | |
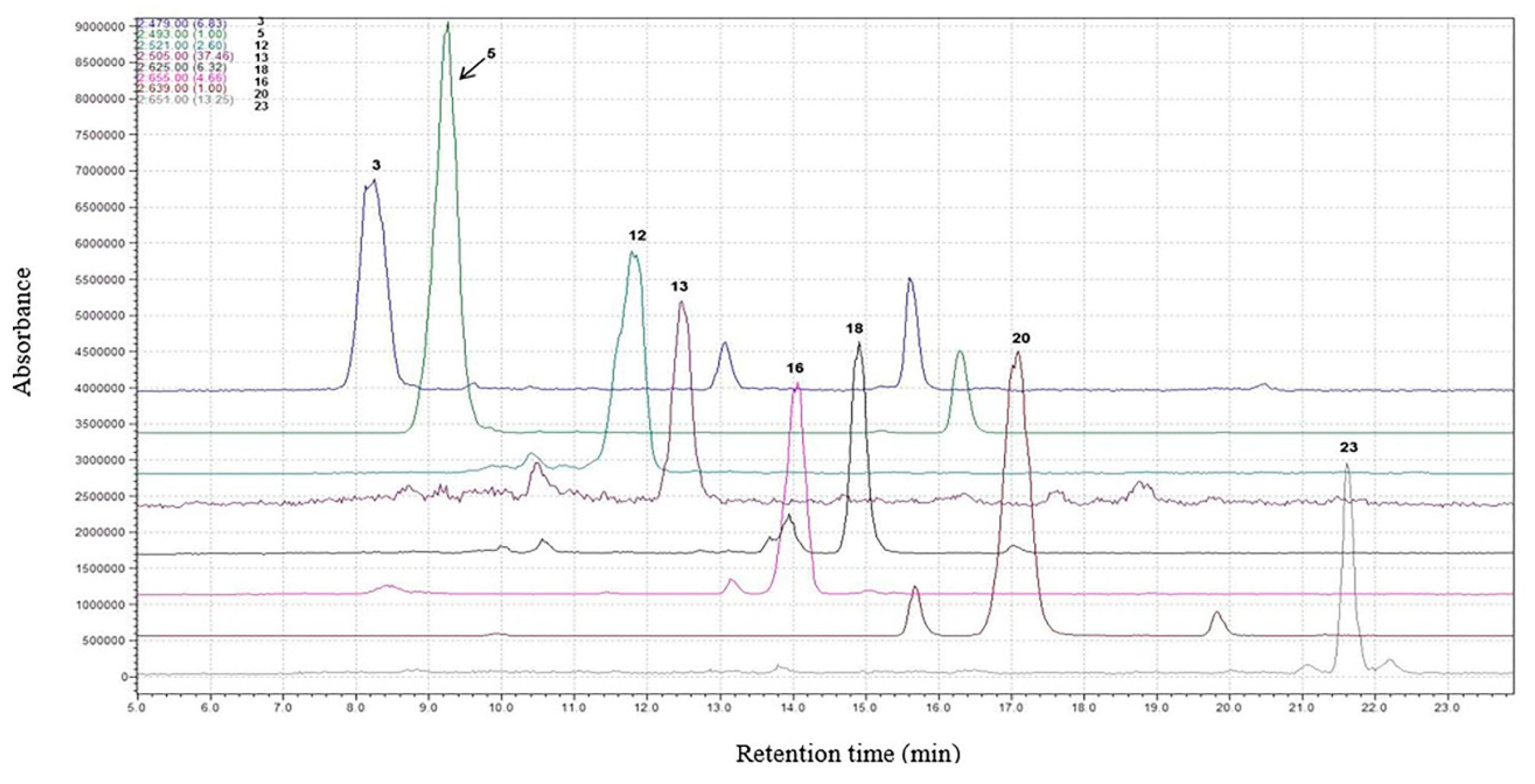
| Peak No. | Identity | tr (min) | m/z (M+) | Concentration (mg 1000 g−1 of DW) * | Concentration (mg·L−1 of Wine) * |
|---|---|---|---|---|---|
| Simple glucosides | |||||
| 1 | Delphinidin-3-O-glucoside | 7.14 | 465 | 4.0 ± 0.2 | 9.6 ± 0.7 |
| 2 | Cyanidin-3-O-glucoside | 7.95 | 449 | - | 2.9 ± 0.1 |
| 3 | Petunidin-3-O-glucoside | 8.25 | 479 | 89.0 ± 5.7 | 76.8 ± 5.0 |
| 4 | Peonidin-3-O-glucoside | 9.06 | 463 | 51.7 ± 3.7 | 26.4 ± 1.7 |
| 5 | Malvidin-3-O-glucoside | 9.27 | 493 | 4222 ± 178 | 342.0 ± 14.0 |
| Acetyl glucosides | |||||
| 6 | Dp-gls-pyruvate derivative | 7.53 | 533 | - | 6.0 ± 0.5 |
| 7 | Pt-gls-pyruvate derivative | 8.67 | 547 | 1.1 ± 0.1 | 2.4 ± 0.1 |
| 8 | Dp-3-(6″-acetylglucoside) | 9.72 | 507 | 24.9 ± 1.5 | 7.6 ± 0.5 |
| 9 | Mv-3-gls-pyruvate (Vitisin A) | 9.84 | 561 | 54.0 ± 3.2 | 10.7 ± 0.9 |
| 10 | Vitisin B (Mv derivative) | 10.20 | 517 | 1.0 ± 0.1 | 8.5 ± 0.7 |
| 11 | Cn-3-(6″-acetylglucoside) | 10.47 | 491 | 332.1 ± 12.5 | 3.3 ± 0.2 |
| 12 | Pt-3-(6″-acetylglucoside) | 10.92 | 521 | 423.3 ± 27.2 | 79.2 ± 5.0 |
| 13 | Pn-3-(6″-acetylglucoside) | 12.48 | 505 | 6.4 ± 0.5 | 29.9 ± 1.9 |
| 14 | Mv-3-(6″-acetylglucoside) | 12.57 | 535 | 4381 ± 306 | 273.7 ± 16.1 |
| Cinnamoyl glucosides | |||||
| 15 | Dp-3-(6″-p-coumaroylglucoside) | 12.93 | 611 | 7.8 ± 0.8 | 5.7 ± 0.4 |
| 16 | Mv-3-(6″-caffeoylglucoside) | 14.07 | 655 | 95.3 ± 6.2 | 2.2 ± 0.2 |
| 17 | Cn-3-(6″-p-coumaroylglucoside) | 14.67 | 595 | 1.3 ± 0.1 | 3.4 ± 0.3 |
| 18 | Pt-3-(6″-p-coumaroylglucoside) | 14.91 | 625 | 55.7 ± 3.1 | 15.7 ± 0.9 |
| 19 | Pn-3-(6″-p-coumaroylglucoside) | 17.01 | 609 | 84.7 ± 6.1 | 27.5 ± 1.7 |
| 20 | Mv-3-(6″-p-coumaroylglucoside) | 17.16 | 639 | 2534 ± 142 | 98.3 ± 6.9 |
| Pyroanthocyanins | |||||
| 21 | Mv-3-(6″-acetylglucoside) pyruvate | 10.68 | 603 | 10.0 ± 0.8 | 7.6 ± 0.5 |
| 22 | Mv-3-glucoside-ethyl-catechin | 11.19 | 809 | 8.5 ± 0.6 | 2.1 ± 0.2 |
| 23 | Mv-3-(6″-acetylglucoside)-4-vinylphenol | 21.66 | 651 | 54.9 ± 4.0 | 2.9 ± 0.2 |
| Total (g/kg) | 12.44 | 1.04 | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trikas, E.D.; Papi, R.M.; Kyriakidis, D.A.; Zachariadis, G.A. A Sensitive LC-MS Method for Anthocyanins and Comparison of Byproducts and Equivalent Wine Content. Separations 2016, 3, 18. https://doi.org/10.3390/separations3020018
Trikas ED, Papi RM, Kyriakidis DA, Zachariadis GA. A Sensitive LC-MS Method for Anthocyanins and Comparison of Byproducts and Equivalent Wine Content. Separations. 2016; 3(2):18. https://doi.org/10.3390/separations3020018
Chicago/Turabian StyleTrikas, Evangelos D., Rigini M. Papi, Dimitrios A. Kyriakidis, and George A. Zachariadis. 2016. "A Sensitive LC-MS Method for Anthocyanins and Comparison of Byproducts and Equivalent Wine Content" Separations 3, no. 2: 18. https://doi.org/10.3390/separations3020018