1. Introduction
This paper intends to give an overview of research results published recently in the field of portable electromigration devices. The time period covered is from 2013 until mid-2015, but other examples not covered in previous reviews may be discussed as well. Under the term electromigration devices are understood various capillary electrophoresis systems and microfluidic devices, which are both miniaturized and, therefore, are well suited for the development of portable equipments.
Previously published and nice reviews may serve as background reading material. Ryvolova
et al. [
1] discussed portable capillary-based electrophoresis, while microfluidic chips were not included in this review. Lewis
et al. gave an overview of portable non-chip-based capillary electrophoresis (CE) systems, portable chip-based CE systems, and put a special emphasis on
in situ CE systems [
2]. The literature was reviewed until 2012. Kaljurand presented a review with a special focus on the analysis of samples in harsh environments [
3]. Indeed, the instrumentation used for field, and even extraterrestrial analysis, needs to withstand inhospitable conditions in terms of temperature, vibration, or radiation. It certainly benefits from portability. The author goes deeper into the requirements for the instrumentation, and notes that more work is needed in this emerging field of separation science in harsh environments. Nuchtavorn
et al. recently described applications from the period 2010–2014 dealing with microchip electrophoresis in biomedical analysis. This review also paid attention to portable devices [
4].
In general, the aim of portability of analytical instrumentation is to make measurements possible outside the laboratory. The instrument should, therefore, be lightweight and have small dimensions; it should be easy to transport, and preferably independent of mains power which implies the use of batteries. The operational lifetime of a device depends on the current running through the capillary or microfluidic channel. In this respect, lower currents result in lower power consumption and, thus, longer operational lifetime [
2]. For example, a substantial advantage of microchip electrophoresis is that it requires lower voltages due to the shorter separation channels [
4]. Microfluidics can also implement ready to use, disposable microfluidic chips, which is an additional asset [
5]. CE is most presumably one of the techniques best suited for field and on-site analysis, because it can easily be miniaturized, the start-up time is short and CE analysis times are among the shortest one can obtain [
6]. The instrument should be robust and mechanically rigid in order to withstand transport and in-the-field operation. There may also be special demands on the detection: microfluidic electrophoresis devices for instance, require a miniaturized and sensitive detection system [
5]. In addition, in many cases the dimensions of the high voltage power supply and acquisition system used together with microchips, are not in concordance with the features of the microchips, which remains a drawback to be solved in order to allow
in situ analysis with a fully portable system [
5].
The typical application areas, in which portability is a specific asset, are point-of-care diagnostics (the so-called clinical analysis), in-the-field measurements for environmental purposes, detection of explosives or chemical warfare agents in the frame of terrorist actions, forensic analysis in a crime scene setting, and food analysis outside the laboratory.
This manuscript first looks into the advances achieved in instrumentation (see
Section 2). This is followed by a discussion on recent applications (
Section 3). There is not necessarily a focus on the complete autonomy of the system, which would be needed for
in situ operations where the equipment must be able to function without a user being present [
2].
2. Advances in Instrumentation
Portable electromigration systems consist of a separation part and a detection part. In order to improve the portability of the system, the device should be miniaturized, which is perfectly achievable through CE capillaries and microfluidic channels. However, this miniaturization has implications for the detection, which should remain sensitive in spite of the small channels being used. Therefore, several recent developments in the field of detection in portable CE and microchip capillary electrophoresis (MCE) will be discussed.
It is striking that a lot of studies have been performed using capacitively-coupled contactless conductivity detection (C
4D). This may be the detection method of choice for portable systems, in view of its low power consumption, the possibility for miniaturization, high versatility, and ease of construction and operation [
7]. It also allows the use of narrow capillaries that usually give better separation efficiencies.
Section 3 highlights a number of applications using C
4D as a detection mode. Nguyen
et al. describe a simple semi-automated portable capillary electrophoresis instrument with C
4D detection [
7]. In this device, the capillary is rinsed by pneumatic operation. Sample injection is performed by manual siphoning. For the first time, a miniaturized commercial high-voltage (HV) power supply was employed for the construction of a portable CE system. Due to its simplicity, the instrument is compatible with circumstances in which there are financial constraints [
7].
The instrument is inexpensive, simple in construction, and can be assembled with little effort. In comparison to the automated versions described by the same group before, this instrument allows use of much smaller sample volumes, which is an extra advantage. The system is yet to be used for on-site applications, but it may already serve as a low cost instrument in the laboratory.
Table 1 gives an overview of some of the typical characteristics of the systems discussed in this review.
Another detection technique with extremely low power consumption and space requirements (size 25 mm × 17 mm) is the capacitance-to-digital conversion technology (CDC) [
8]. It is similar to C
4D in that it also measures complex impedance, but the signal conversion differs from conventional analog-to-digital modulation. The presented single chip detector was integrated into a commercially-available CE cassette, and revealed a limit of detection (LOD) of 1 µM for sodium and 1.6 µM for potassium ions. It can compete with known miniaturized C
4D and UV detectors in terms of sensitivity, resolution, size, and power requirements. The low working frequency of this detector minimizes stray capacitance (
i.e., the capacitance associated with direct coupling between the electrodes) and it may be used in indirect mode with an ascorbic acid background electrolyte. In addition, a high conductivity borate buffer can be used as a background electrolyte in combination with this detector, which is not the case with a C
4D detector [
8].
Table 1.
Portable electromigration devices.
Table 1.
Portable electromigration devices.
| Size | Weight | Voltage Applied | Injection | Operational Lifetime | Electrophoresis HV Supply | Detector | Power Source | Reference |
|---|
Fluidic part: plexiglass block (3 cm × 2 cm × 2 cm);
All components integrated into a Perspex case of dimensions 40 cm (w) × 28 cm (d) × 21 cm (h) | 6 kg | 20 kV | Hydrodynamic injection by siphoning directly from the sample vial | | Miniature commercial HV supply module of dimensions 120 mm (l) × 38 mm (w) × 25 mm (h) and weight 200 g | Miniaturized C4D detector built in-house | For electrophoretic and fluidic parts: Li battery pack of 14.8 V with capacity 6.6 Ah.
For C4D circuitry: two Li-ion batteries with capacity 2.8 Ah each | [7] |
| 165 × 150 × 85 mm | - | 3 kV | Application of voltage along the injection channel of the microchip | - | Has one channel with four outputs and was designed for unpinched injection and separation | Amperometry based on three Ti/Pt thin film electrodes | Battery-powered instrument that can be connected to the computer by means of USB-RS232 cable or Bluetooth® | [5] |
| 12 × 12 × 8 Inches | Total weight: 28 lb | 3 kV | - | - | Four HV power supplies (one 3 kV, three 1 kV) | Scanning fluorescence detector designed in-house | Instrument supplied with 12 V through separate 120 V power supply | [9] |
| 15 cm × 5 cm × 8 cm | 0.4 kg | 150 V | Application of voltage along the injection channel of the microchip | - | - | LIF | The system consumes appr. 300 mW and is powered from the USB 5 V supply. | [10] |
| 45 × 35 × 15 cm (w × d × h) | 8 kg | 25 kV | Injection interface: 3 cm × 2 cm × 2 cm | 9 h in the battery powered mode | Two high voltage modules | C4D | Built-in rechargeable lithium-ion batteries.
Battery pack of 14.8 V with capacity 6.6 Ah.
For C4D circuitry: two Li-ion batteries with capacity 2.8 Ah each | [11] |
| 14 × 25 × 8 cm (w × l × h) | 1.2 kg | 5 kV | Application of voltage along the injection channel of the microchip | 10 h | Miniaturized dual polarity high voltage power supply | Dual top-bottom C4D | Two lead acid batteries | [12] |
| 450 × 150 × 350 mm (w × h × d) | 8 kg | 25 kV | Sample is pumped through the grounded interface and partially pumped into the capillary. | As in [11] | As in [11] | C4D | As in [11] | [6] |
| 27 × 44 × 13 (w × l × h) | 8 kg; alm PCR: 350 g | 10 kV | Electrokinetic injection | - | Commercially available | LIF | - | [13] |
| 310 × 220 × 260 mm (w × h × d) | - | 15 kV | Hydrodynamic injection by siphoning | - | Commercially available | C4D | The equipment runs on mains power as well as battery power | [14] |
Another group reported on a portable fluorescence detector that was integrated into a portable genetic analyzer [
15]. Fluorescence detection is one of the detection modes well suited for measurements in small optical path lengths, because of its high intrinsic sensitivity. The overall portable genetic analysis system consists of a Polymerase Chain Reaction (PCR) and CE part with glass-based microchip, a miniaturized operational hardware, a cooling fan, a fluorescence detector and a laptop computer. Single nucleotide polymorphism (SNP) testing has a high potential for point-of-care testing, and therefore this portable device is quite useful. It may be applicable in point-of-care DNA diagnostics, food safety testing and pathogen detection. This instrument was applied for on-site identification of Korean indigenous beef cattle [
15].
Electrochemical detection in general has proven to be very effective due to its inherent miniaturization, sensitivity, low cost, portability, and compatibility with microfabrication technologies [
5]. In addition to the aforementioned C
4D, amperometry and potentiometry are also commonly employed in combination with MCE. A novel commercially-available portable CE instrument is presented using SU-8/Pyrex microfluidic chips with amperometric detection. This HVStat was used for the first time for urine analysis (see also
Section 3).
Scherer
et al. reported on the design and operation of a portable scanner for high-performance MCE in arrays [
9]. The work was a continuation of previous developments of portable instruments applicable for real-time forensic short tandem repeat analysis outside the laboratory. A compact scanner was developed containing the classical multicolor rotary scanning confocal detection system, together with pneumatic and electronic components to provide a fully integrated microfluidic platform. It is a self-contained instrument featuring the four-color confocal detection system, as well as a round, heated platform to support single channel, as well as 384 channel wafers, four independent temperature control systems with microfabricated heaters, and a total of 28 solenoid valves to realize the fluidic movement by pressure/vacuum. Applications are to be expected in the fields of high-throughput DNA sequencing, on-site human identification in forensics, and pathogen detection in homeland security [
9].
Finally, regarding detection in CE, there has been a report on portable microcoil nuclear magnetic resonance (NMR) detection coupled to CE [
16]. The results demonstrated for the first time that coupling a commercial CE instrument to a portable NMR instrument is feasible and can provide a low-cost method to obtain structural information on microliter samples in a non destructive way. The radiofrequency (RF) microcoils used in this form of NMR, have been downsized. In view of the fact that the sensitivity improves with decreasing coil diameter, improved mass sensitivity may be observed. However, in view of the fact that concentrations required are still relatively high, non-standard CE conditions had to be incorporated. 200 µm i.d. capillaries had to be used to increase the amount of analyte in the NMR detection window, which also leads to poor peak resolution. Moreover, the CE voltage induces local magnetic fields that cause a distortion of the magnetic field homogeneity produced by the RF coil, with concomitant line broadening and poor signal to noise ratio in the NMR signals. In order to avoid this effect, the voltage was turned off during NMR data acquisition. The experiments were thus performed in a stop flow mode, with mobilization via pressure or voltage. This was applied on fluorinated compounds by using
19F NMR, thus avoiding derivatization. Full portability would be achieved by coupling this portable NMR system to a portable CE instrument, offering also a reduced footprint [
16].
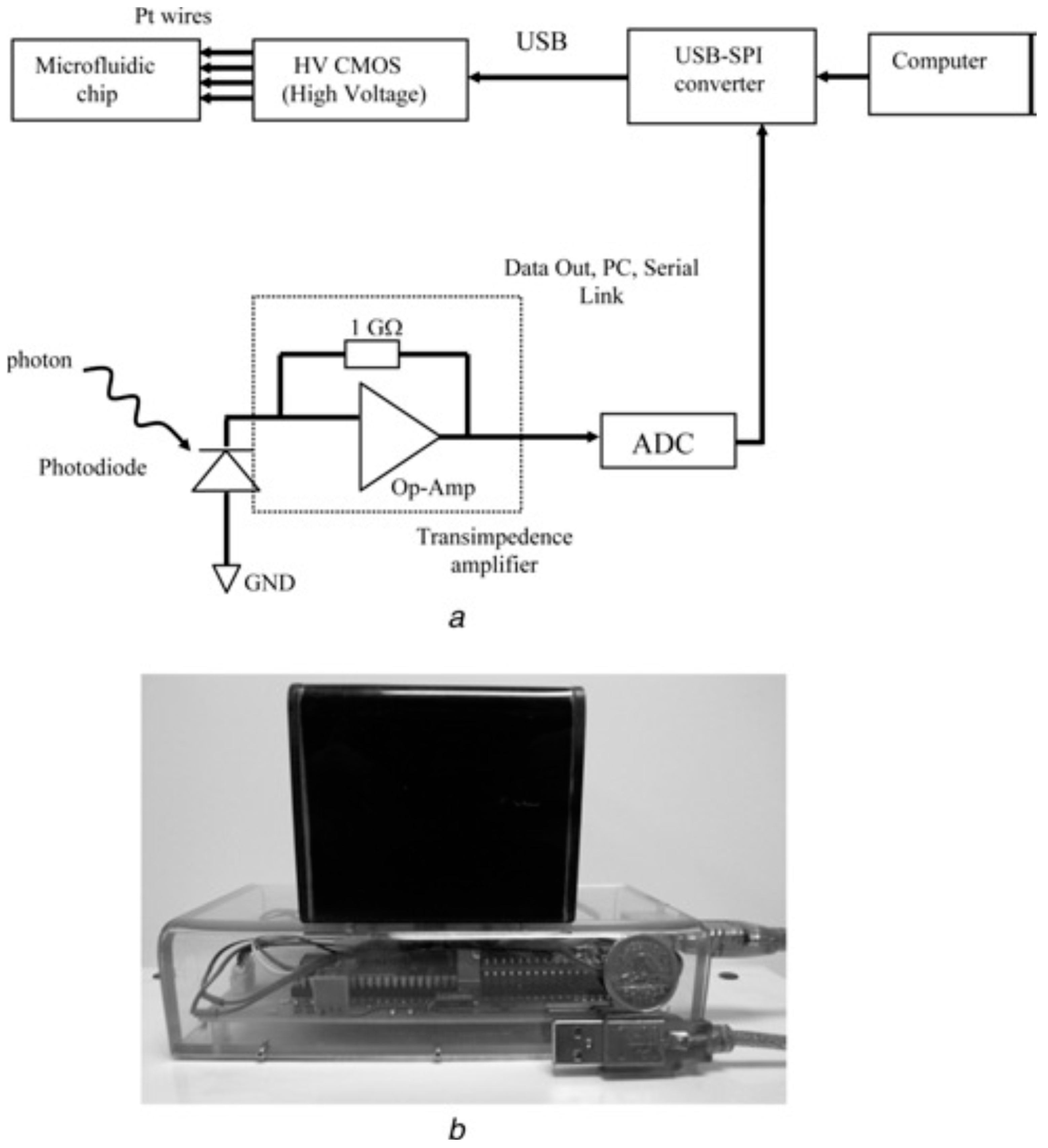
Recently, an inexpensive, compact, and fully-portable lab-on-a-chip-based CE instrument was described [
10]. A previously-developed microelectronic chip for high-voltage generation, switching, and interfacing was now integrated with a microfluidic chip, a solid state laser, filter, lens, and a few other electronic components (see
Figure 1).
Figure 1.
Handheld CE platform. (
a) Block diagram representing the architecture of the CE platform comprised of an USB-SPI converter, HV CMOS chip, an ADC and a photodiode (PD). (
b) The handheld CE platform that is powered using a USB link connected to a laptop computer. The upper black section is where the CE is performed and the lower section (plexiglass) is the housing for the optical components and the HV-related components. Reproduced by permission of the Institution of Engineering and Technology. Full acknowledgment to Kaigala, G.V.
et al. [
10]. Copyright Institution of Engineering and Technology, 2009.
Figure 1.
Handheld CE platform. (
a) Block diagram representing the architecture of the CE platform comprised of an USB-SPI converter, HV CMOS chip, an ADC and a photodiode (PD). (
b) The handheld CE platform that is powered using a USB link connected to a laptop computer. The upper black section is where the CE is performed and the lower section (plexiglass) is the housing for the optical components and the HV-related components. Reproduced by permission of the Institution of Engineering and Technology. Full acknowledgment to Kaigala, G.V.
et al. [
10]. Copyright Institution of Engineering and Technology, 2009.
It was the first demonstration of an inexpensive (total component cost of less than $175 USD), microelectronic and microfluidic chip based system. The instrument is completely controlled and powered through a USB link from a laptop computer, thanks to its reduced power requirements. This platform has been used for analysis of clinically-relevant PCR products, and should allow point-of-care medical diagnostics [
10].
The group of Peter Hauser reported on the establishment of a portable CE instrument with automated injector and C
4D detection [
11]. As stated above, electrochemical detection is extremely well suited for portable CE because of the straightforward miniaturization and low power format. C
4D can be considered universal for all ionic species. C
4D detectors are small, cheap, lightweight, and consume only little electrical power [
6]. The fact that the electrodes are located outside the capillary, is quite advantageous in the sense that the detector can be easily constructed and operated. In this report the authors address the injection of samples, which has been a weak point of the portable CE instruments until then, as the injection process required very delicate manual operations needing careful manipulation [
11]. The automated system, thus, also contributes to a better repeatability of injection as opposed to manual pressure injection with a syringe. The key element in the setup is a splitter through which the sample is passed automatically (see
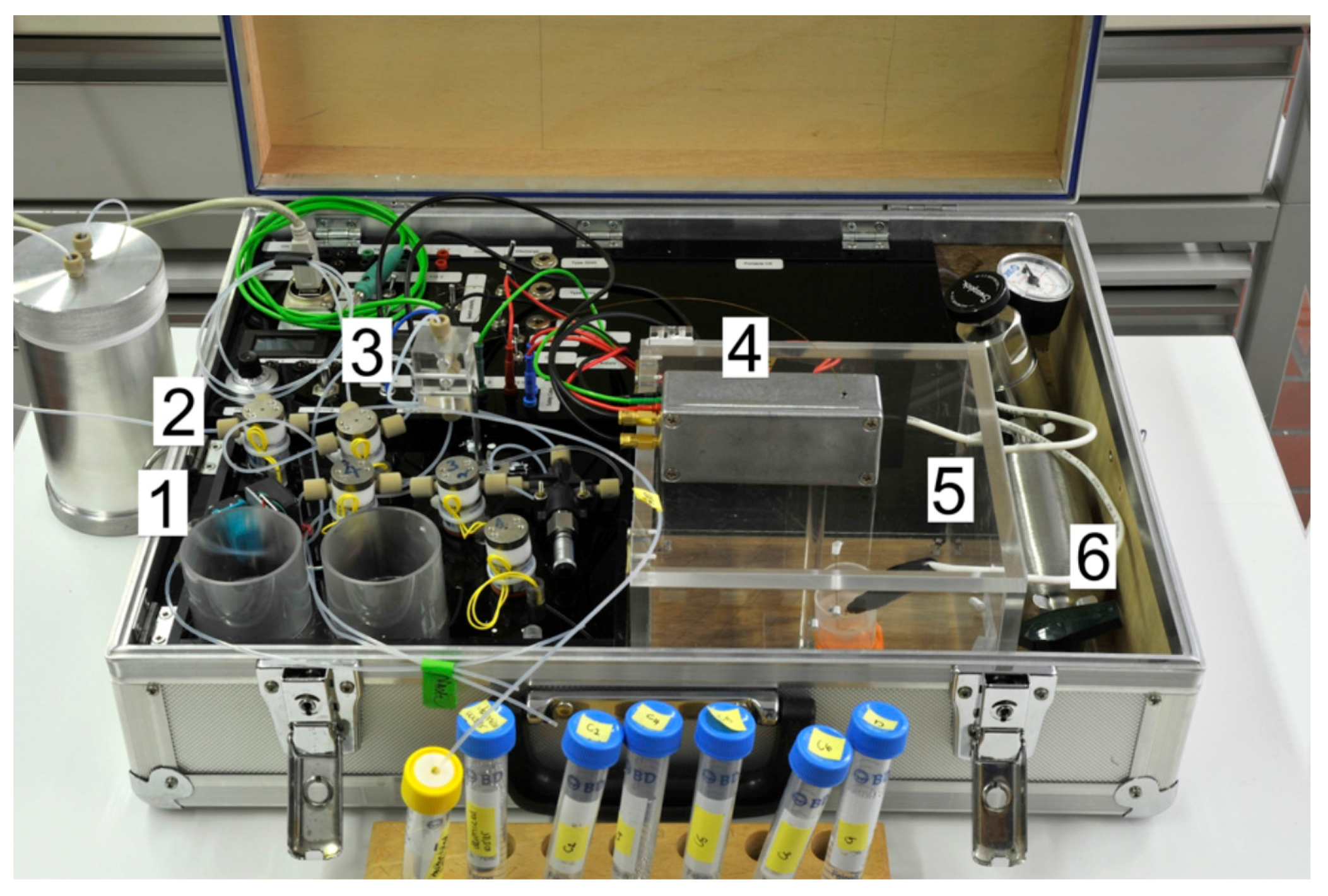
Figure 2).
Figure 2.
Photograph of the instrument. (
1) Membrane pump; (
2) valves; (
3) splitter; (
4) detector; (
5) safety cage for application of high voltage; (
6) pressurized air. Reprinted with permission from Mai, T.D.
et al. [
11]. Copyright 2013 American Chemical Society, 2013.
Figure 2.
Photograph of the instrument. (
1) Membrane pump; (
2) valves; (
3) splitter; (
4) detector; (
5) safety cage for application of high voltage; (
6) pressurized air. Reprinted with permission from Mai, T.D.
et al. [
11]. Copyright 2013 American Chemical Society, 2013.
The use of fixed pressurization with a small membrane pump and computer-controlled timing precludes the variations of manual operation. In view of the automated aspiration of the samples, the instrument also has the potential to be set up for unattended monitoring operations. The performance of this device was illustrated with the separation of four anions within 17 s, showing its ability for fast separations. Low limits of detection could be achieved for nitrite (determination below 1 µm). High peak capacity could be obtained with complex samples such as soft drinks. Finally, the operation in the field was demonstrated with the determination of phosphate at a sewage treatment plant. It is interesting to note that practical difficulties may arise during on-site measurements. Freshly collected wastewater samples may for instance contain some dissolved gases. As normally no degassing can be carried out outdoor, the gas bubbles may influence the precision of injection [
11].
It is worthwile to shortly address the injection of samples in portable instruments. Ideally, sample introduction should be easy to perform, robust and compatible with portability, and should yield repeatable results. Several options are available, each having advantages and disadvantages. Up until recently the injection was performed mostly by conventional injection procedures (electrokinetic or hydrodynamic injection) [
17]. Pressure may be applied by pumps or with the use of syringes. Though pumps will give a better repeatability, they jeopardize the portability of the system because of an increase in overall size and weight of the instrument [
1]. It needs to be realized that it may sometimes be tedious to perform injections from microliter sample vials in a field setting [
17]. Various flow splitting devices have been introduced which simplify the injection and yield better repeatability of peak areas. Flow injection is amenable to automation and high throughput applications.
Applying C
4D, a MCE instrument has been developed with a special C
4D configuration, namely a replaceable cell cartridge implementing dual top-bottom C
4D [
12]. The two electrodes of the detection cell need to be in close proximity of the microfluidic channel. The cell capacitance should be as large as possible for maximum sensitivity. The stray capacitance should be minimal. The gap distance between the excitation and pickup electrodes is critical because a compromise needs to be found between a large gap size for increased sensitivity and enough resolution between the analyte peaks. In dual C
4D, replaceable C
4D cells were designed with variable electrode gap sizes and well-defined electrode geometries. The cells are housed in such a way that minimal noise level and stray capacitance are obtained.
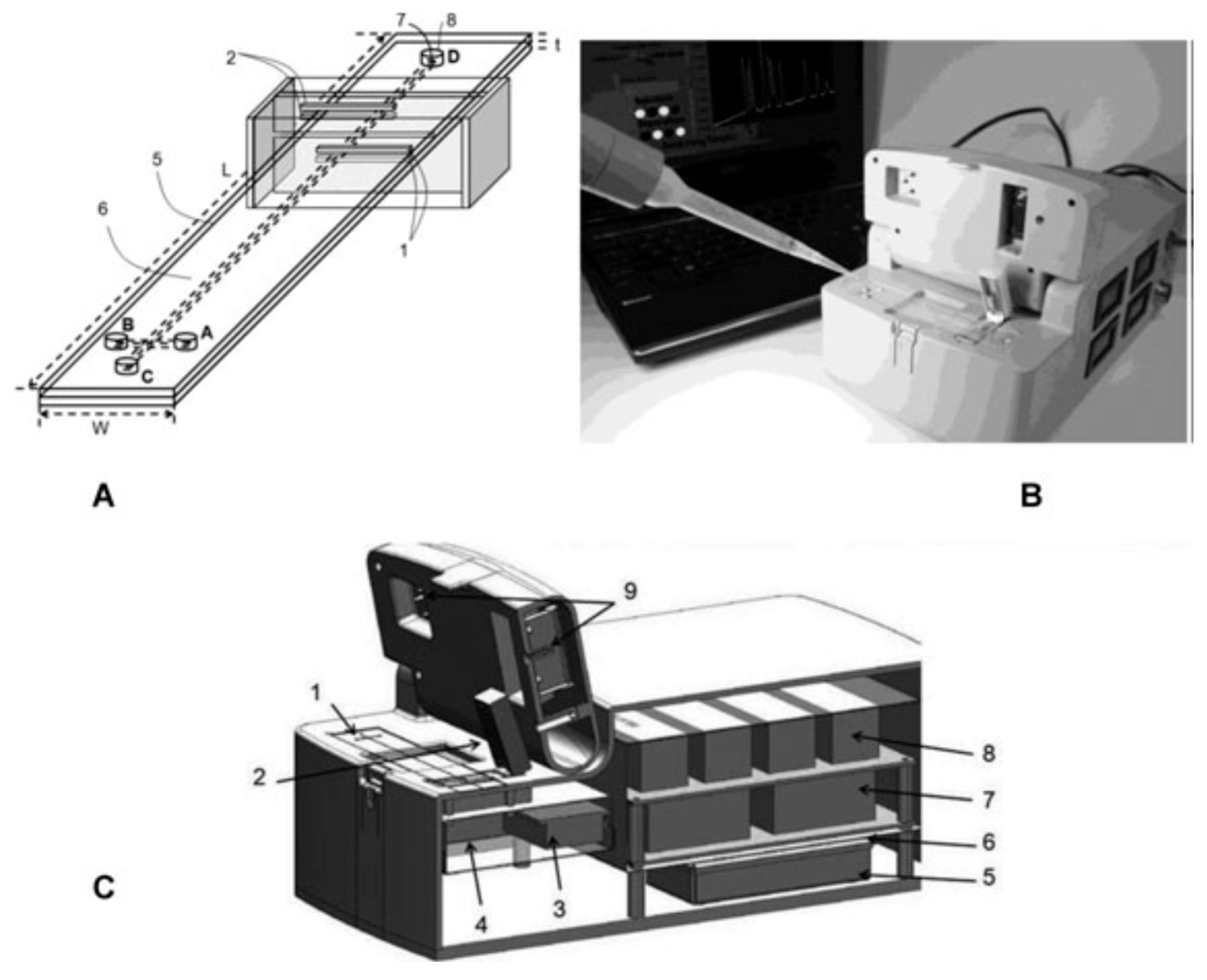
Figure 3 illustrates the setup of the instrument.
Figure 3.
(
A) Microchip layout with dC
4D cell: (1) Excitation electrodes; (2) Pick-up electrodes; (3) Shielded box; (4) Ground plane; (5) Microchip (
L = 10 cm,
W = 2 cm,
t = 125 µm); (6) Cross microchannels with 50 × 50 µm
2 square cross sections; injection channel (sample reservoirs: A → B) 1 cm long; separation channel (buffer reservoirs: C → D) 9 cm long; (7) Imprinted reservoirs connected to microchannels 0.5 mm diameter; (8) Punched through holes into top lead for fluidic inlets-outlets 2 mm diameter; (
B) Optical photograph and schematic diagram of the E-dC
4D instrument with hardware and electrophoresis components; and (
C) Schematic showing the detailed view of the instrument: (1) Plastic chip; (2) dC
4D cell in cartridge format; (3) Pickup amplifier and its electronic circuit; (4) Signal generator; (5) Signal processing electronics; (6) Data acquisition (DAQ) card; (7) High-voltage power supplies; (8) High-voltage relays and optocouplers; (9) Electrophoresis compartment. Reproduced with permission from Wiley-VCH, 2013 [
12]. Copyright Wiley-VCH, 2013.
Figure 3.
(
A) Microchip layout with dC
4D cell: (1) Excitation electrodes; (2) Pick-up electrodes; (3) Shielded box; (4) Ground plane; (5) Microchip (
L = 10 cm,
W = 2 cm,
t = 125 µm); (6) Cross microchannels with 50 × 50 µm
2 square cross sections; injection channel (sample reservoirs: A → B) 1 cm long; separation channel (buffer reservoirs: C → D) 9 cm long; (7) Imprinted reservoirs connected to microchannels 0.5 mm diameter; (8) Punched through holes into top lead for fluidic inlets-outlets 2 mm diameter; (
B) Optical photograph and schematic diagram of the E-dC
4D instrument with hardware and electrophoresis components; and (
C) Schematic showing the detailed view of the instrument: (1) Plastic chip; (2) dC
4D cell in cartridge format; (3) Pickup amplifier and its electronic circuit; (4) Signal generator; (5) Signal processing electronics; (6) Data acquisition (DAQ) card; (7) High-voltage power supplies; (8) High-voltage relays and optocouplers; (9) Electrophoresis compartment. Reproduced with permission from Wiley-VCH, 2013 [
12]. Copyright Wiley-VCH, 2013.
![Separations 03 00002 g003]()
The figure shows that the front part of the equipment contains the chip in its holder, as well as the electrodes and the detector. The remainder of the equipment components (electronics, data acquisition card, high voltage power supplies, batteries) are situated in the second part.
Figure 4 shows several cartridges with variable electrode gaps. The cell can be opened to place the fluidic chip. When closing the cell, top and bottom are clamped together and in doing so, the pairs of top-bottom excitation and pickup electrodes are aligned with high precision onto the surface of the plastic chip. Using a cell with a narrow gap of 0.5 mm allows reach high resolution, while a 2 mm gap yields a low detection limit. The performance of this device was investigated in three fields: the analysis of ions in water, the analysis of organic acids and preservatives in fruit drinks and the determination of ions in rabbit blood serum and human urine samples [
12].
Figure 4.
(
A) Replaceable dC
4D cartridges of 0.5, 1.0, and 2 mm detection gaps (the distance between the excitation and pickup electrodes along the separation microchannel); (
B) The top-bottom excitation and pickup electrodes with ground plane in between. These components are positioned precisely within a small Faraday cage and isolated by polycarbonate; (
C) The cartridge cell with three pins to contact to input signal, pickup signal, and ground is plugged in from the top. The top contains top excitation electrode, pickup electrode, and ground which are aligned to face and contact the bottom equivalents upon closing. Reproduced with permission from Wiley VCH 2013 [
12]. Copyright Wiley VCH, 2013.
Figure 4.
(
A) Replaceable dC
4D cartridges of 0.5, 1.0, and 2 mm detection gaps (the distance between the excitation and pickup electrodes along the separation microchannel); (
B) The top-bottom excitation and pickup electrodes with ground plane in between. These components are positioned precisely within a small Faraday cage and isolated by polycarbonate; (
C) The cartridge cell with three pins to contact to input signal, pickup signal, and ground is plugged in from the top. The top contains top excitation electrode, pickup electrode, and ground which are aligned to face and contact the bottom equivalents upon closing. Reproduced with permission from Wiley VCH 2013 [
12]. Copyright Wiley VCH, 2013.
Sáiz
et al. improved a previous portable CE machine [
11] by introducing a number of new features [
6]. First of all, fully automated pre-run capillary conditioning was made possible during which four different solutions could be used. This allows avoid manual operations for rinsing the capillary. Second, a new redesigned grounded interface allowed sample injection by hydrodynamic sample splitting. At the same time, electrical power consumption was minimized. Third, a normally open valve as V1 was used to avoid bad repeatability of migration times as opposed to a normally closed valve before the separation step.
3. Recent Applications
In order to be deployable outside the lab, sample preparation should be minimal, little consumables should be needed, the instrument should be easy to use, the analysis times should be low and the analytical validation parameters should comply with the specifications.
In this section some recent applications in the biomedical, forensic, and environmental fields are highlighted.
In the biomedical field, Fernandez-la-Villa applied the above described instrument with amperometric detection for clinical analysis of urine [
5]. The urine samples were diluted with the background electrolyte, filtered and injected directly onto the microchip for analysis. Uric acid could be determined within 90 s. It was baseline separated from interfering compounds such as ascorbic acid and paracetamol. One single microchip could be used during more than 20 days, performing over 900 runs.
The control of β-agonists contents in pig feed and pharmaceutical samples may benefit from quick and inexpensive on-site sampling and analysis. Nguyen applied their equipment for the analysis of salbutamol, metoprolol, and ractopamine [
7]. In order to cope with the fluctuation of migration times due to changing environmental temperatures, spiking with standards was performed for confirmation. The standard addition method was applied for quantitation.
Lim
et al. improved a previously reported system in terms of sensitivity, robustness, and heat dissipation capacity [
13]. The equipment combines a palm PCR device to amplify samples on the spot with a portable CE.
The laser power of the LIF detector was doubled in comparison with the previous version, yielding a 10-fold enhanced sensitivity. The internal structure was reinforced for improved robustness. A fan was installed on the side of the aluminum housing for heat dissipation. The authors then applied this equipment to the diagnosis of influenza A (H1N1) virus. In the event of a flu outbreak, portable instruments can be powerful weapons in early and on-site diagnosis. The background electrolyte typically makes use of a polyvinylpyrrolidone sieving gel matrix for separation of DNA fragments of 100 to 3000 base pairs (bp), and the diagnostic fragments are situated between 100 and 200 bp [
13].
The field of forensic analysis may also benefit from having systems available for fast analysis of suspicious samples. Sáiz
et al. focused their attention on scopolamine [
18]. This alkaloid is clinically used, but also abused because of its influence on neurotransmission pathways concerning memory. It can thus cause amnesia, but in addition can also block free will. For this reason it is used in robberies and sexual assaults. Due to its hallucinogenic effects, it is also used as a recreational drug. Scopolamine is rapidly eliminated from the body, and cannot be detected anymore in the organism after 24 h. Portable CE is especially suited for this type of rapid analysis. Typical items used for recreational and predatory purposes include an infusion of
Datura stramonium seeds, moisturizing creams, and alcoholic beverages containing scopolamine. The authors also developed a simple sample pre-treatment to keep analysis times short. Finally, rapid screening of evidence could be performed within 3.5 min. C
4D was proven to be an excellent detector for this application [
18]. This paper made use of the purpose built portable CE equipment that was described above [
6].
Lloyd
et al. used a commercial MCE device to investigate synthetic cathinone seizures [
19]. These compounds typically occur on the illicit drug market. Visually-similar tablets may contain varying constituents in different proportions. It would be useful to have a rapid screening method for cathinone containing tablets. The elaborated method allowed a comparative screening through the MCE profiles rather than an identification, but in conjunction with gas chromatography coupled to mass spectrometry (GC-MS) as a primary identification technique, larger numbers of samples can be analyzed without increasing the burden on forensic laboratories. Ongoing research includes the use of chemometric tools as well as automated procedures to improve the screening abilities of this MCE method [
19].
The instrument described above [
6] was applied to the determination of nitrogen mustard degradation products. The area of chemical warfare necessitates the availability of portable tools for field analysis, so as to achieve effective and reliable detection of chemical warfare agents [
6]. Field analysis in general allows avoid sample transport and storage. Nitrogen mustards have never been used in combat up until now and their production, use, and stockpiling are prohibited. No antidote exists for exposure to these substances. The use of nitrogen mustards in chemical warfare can be detected by monitoring degradation products formed by hydrolysis in the presence of water. These products carry amino functional groups, which may interact with silanol groups on the fused silica capillary wall. Therefore, a polymeric wall coating was introduced, and the capillary coating procedure was performed at higher pressures and temperatures yielding the best repeatability of migration times. The degradation products could be determined in spiked water samples from different origins [
6].
Interestingly, portable CE has also been applied to the fingerprinting of explosive residues remaining after an explosion initiated by inorganic as well as organic explosives [
20]. After, for instance terrorist attacks, it is important to obtain information about the type of explosive which has been used, because this may lead to quicker identification of aggressors and may prevent other attacks. The novel aspect of this paper resides in the study of various surfaces (sand, concrete, metal plate) on which the explosions were carried out. Commercial organic and improvised inorganic explosives were used. Surfaces were wiped postblast according to a standardized procedure, and the samples were analyzed with a previously described CE-C
4D equipment and background electrolyte. Anions and cations could be analyzed in the same run thanks to dual-opposite end injection. However, the blank surfaces could cause some matrix interference due to the presence of, especially, cations. It was therefore imperative to subtract the blank signals from the measured data. It was found that explosives can be identified, either on the basis of a flow chart (for the metal surface) or on the obtained ion patterns that can be arranged by Principal Component Analysis into well defined clusters [
20].
Some interesting applications have also been described in the field of environmental and planetary investigations.
Torres
et al. intended to look into the analysis of sediment porewater because this gives an insight into various biogeochemical processes and cycles [
14]. On-site methods are to be preferred for this kind of investigations, since transportation of sediments may cause changes in their composition. However, on-site analysis involves the collection/extraction of the porewater as well as the analysis. In this paper, the extraction of the sediment porewater could be performed in a convenient and simple way using commercially available microporous polymer tubes. The water samples should then be analyzed immediately to prevent contamination. Indeed, the porewater which is normally not exposed to ambient air, contains various compounds that are prone to oxidation by oxygen in air. In this way, oxygen-sensitive Fe species can be analyzed together with other relevant inorganic ions. For remote areas it is important to have lightweight and simple equipment. This kind of equipment was offered by a portable CE with C
4D detection.
Only minute volumes of sample were required allowing to have low disturbance of the sediment cores and a high spatial resolution. The sediment core of a lake in Switzerland was studied [
14], as well as lake Baikal in Siberia [
21]. In the latter paper, the portable CE instrument with C
4D detector proved to be ideal and reliable for field work even though local working conditions were challenging.
Mora
et al. used a portable MCE device with LIF detection for the analysis of thiols [
22]. This class of compounds is relevant in planetary investigations because they represent a potential biosignature in the search for extraterrestrial life. A set of 12 thiols were fluorescently derivatized enabling limits of detection in the low nM range. Separations were conducted in micellar electrokinetic chromatography mode, allowing analysis of the twelve thiols in less than two minutes following a labeling step of 2 h.