Determination of Morphine and Its Metabolites in Human Urine by Capillary Electrophoresis with Laser Induced Fluorescence Detection Employing On-Column Labeling with a New Boronic Acid Functionalized Squarylium Cyanine Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Buffers, and Sample Solutions
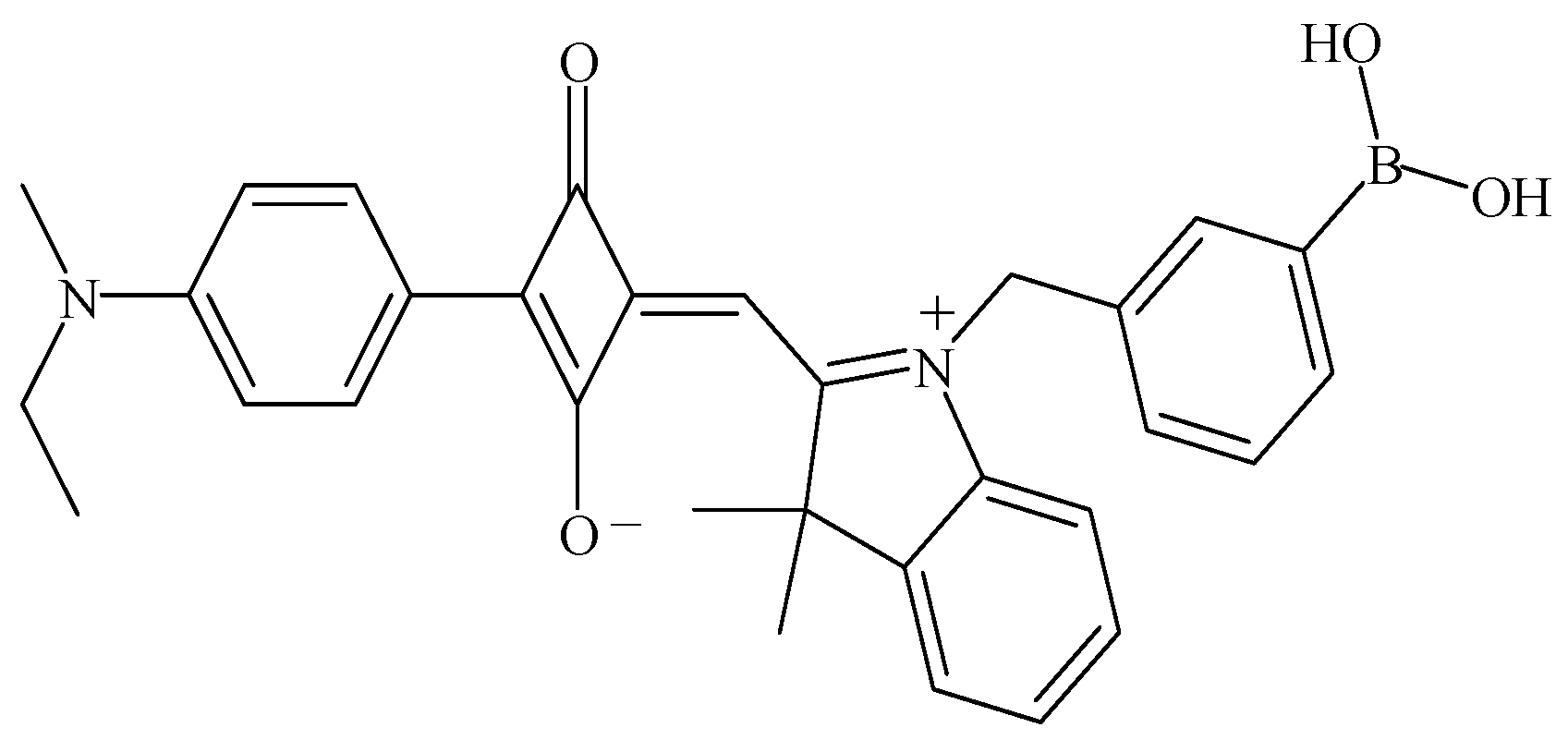
2.2. Human Urine Sample Preparation
2.3. Instrumentation
| Solvent or Buffer | SQ-BA3 Alone | SQ-BA3 with Morphine | SQ-BA3 with M3G | SQ-BA3 with M6G | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λex (nm) | λem (nm) | λex (nm) | λem (nm) | % Fluorescence Enhancement | λex (nm) | λem (nm) | % Fluorescence Enhancement | λex (nm) | λem (nm) | % Fluorescence Enhancement | |
| DMF | 631 | 661 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Ammonium Citrate (pH = 3.50) | 620 | 659 | 621 | 659 | 4.05% | 620 | 660 | 14.33% | 622 | 659 | 3.90% |
| Tris-HCl (pH = 7.50) | 619 | 660 | 621 | 660 | 6.10% | 622 | 661 | 7.79% | 623 | 660 | 6.10% |
| Ammonium Phosphate (pH = 9.50) | 618 | 657 | 619 | 658 | 18.20% | 621 | 659 | 23.45% | 621 | 659 | 6.20% |
| Ammonium Phosphate (pH = 11.30) | 617 | 659 | 619 | 660 | 8.05% | 619 | 659 | 9.52% | 618 | 659 | 4.8% |
3. Results and Discussion
3.1. Spectral Properties of SQ-BA3 Alone and with Morphine, M3G, and M6G

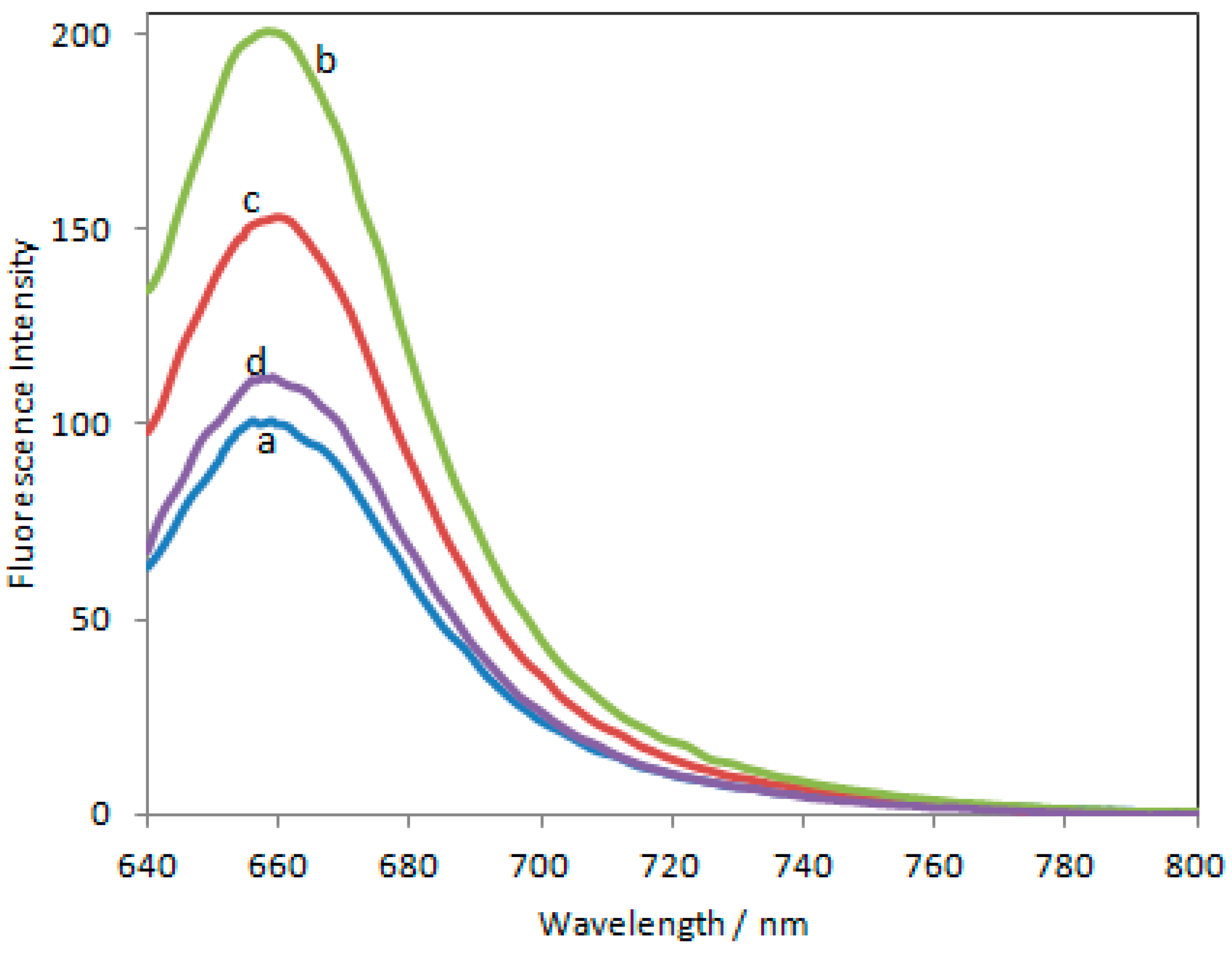
3.2. Stability of SQ-BA3 Solutions
3.3. Optimization of CE-LIF Methods for Morphine and Its Metabolites
| Figures of Merit | Morphine | M3G | M6G | |||
|---|---|---|---|---|---|---|
| Water Matrix | Diluted Urine Matrix | Water Matrix | Diluted Urine Matrix | Water Matrix | Diluted Urine Matrix | |
| Linear Regression (Conc. vs. Peak Area) | y = (1 × 1010)x −1 × 106 | y = (2 × 1010)x −5 × 106 | y = (5 × 1010)x −7 × 106 | y = (3 × 1010)x −5 × 106 | y = (7 × 109)x −1 × 106 | y = (4 × 109)x −2 × 106 |
| Correlation Coefficient, R2 | 0.9974 | 0.9977 | 0.9965 | 0.9962 | 0.9973 | 0.9950 |
| % RSD | 5.34 | 4.47 | 5.35 | 4.61 | 4.46 | 5.89 |
| Linearity range (M) | 2.50 × 10−4–2.50 × 10−3 | 3.50 × 10−4–1.75 × 10−3 | 1.50 × 10−4–1.50 × 10−3 | 2.50 × 10−4–1.25 × 10−3 | 5.00 × 10−4–2.00 × 10−3 | 1.00 × 10−3–1.75 × 10−3 |
| LOD (M) | 1.20 × 10−4 | 1.10 × 10−4 | 8.70 × 10−5 | 9.30 × 10−5 | 1.10 × 10−4 | 1.80 × 10−4 |
| LOQ (M) | 4.10 × 10−4 | 3.80 × 10−4 | 2.90 × 10−4 | 3.10 × 10−4 | 3.80 × 10−4 | 5.90 × 10−4 |
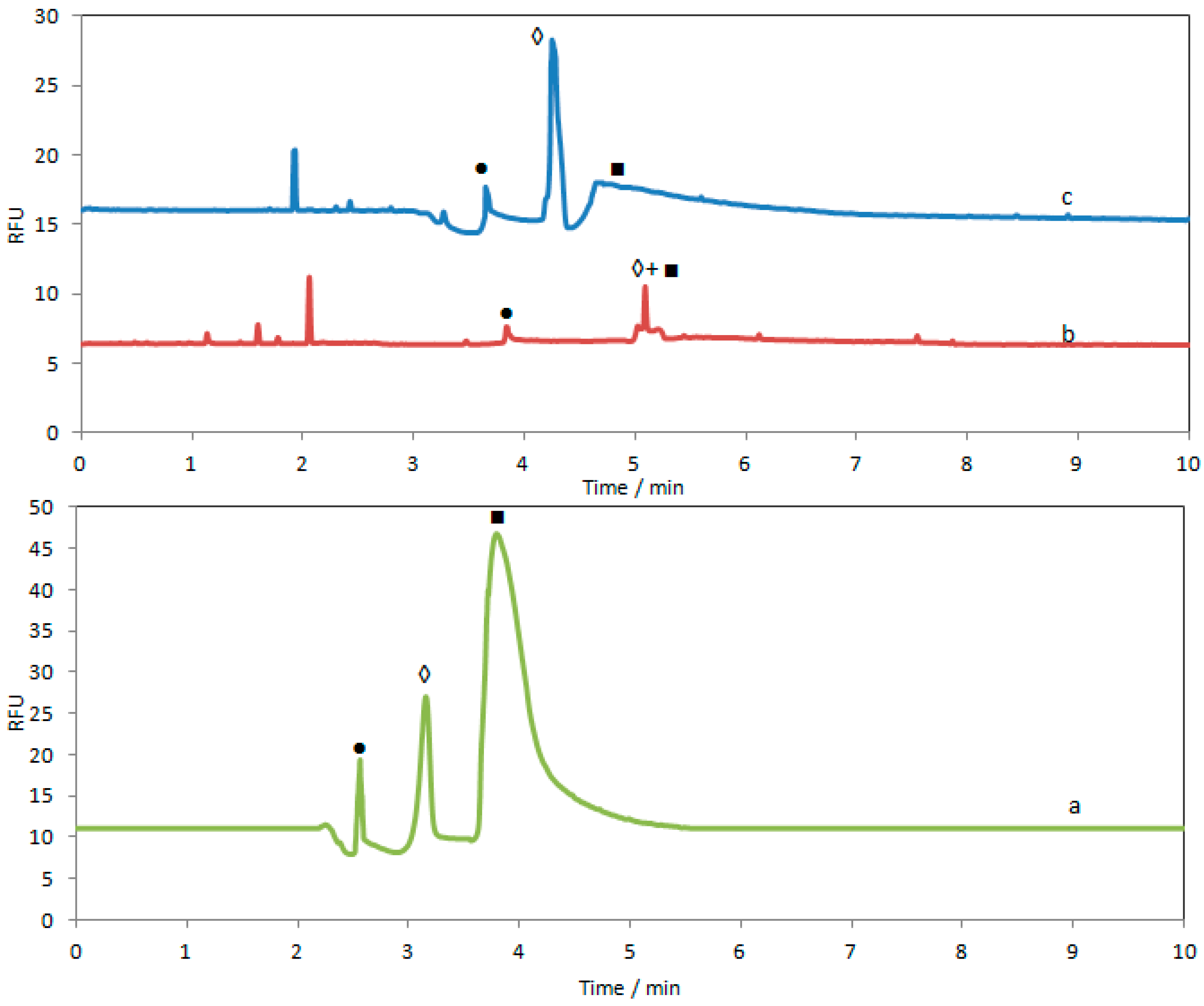
3.4. CE-LIF Analysis of Morphine and Its Metabolites in Human Urine Samples
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coffman, B.L.; Rios, G.R.; King, C.D.; Tephly, T.R. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab. Dispos. 1997, 25, 1–4. [Google Scholar]
- Ulens, C.; Baker, L.; Ratka, A.; Waumans, D.; Tytgat, J. Morphine-6beta-glucuronide and morphine-3-glucuronide, opioid receptor agonists with different potencies. Biochem. Pharmacol. 2001, 62, 1273–1282. [Google Scholar] [CrossRef]
- Stone, A.N.; Mackenzie, P.I.; Galetin, A.; Houston, J.B.; Miners, J.O. Isoform activity and kinetics of morphine-3 and 6-glucuronidation by human UDP-glucuronosyltransferases: Evidence of atypical glucuronidation kinetics by UGT2B7. Drug Metab. Dispos. 2003, 31, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Christrup, L.L. Morphine metabolites. Acta Anaesth. Scand. 1997, 41, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Watt, J.A.; Cramond, T. Morphine-3-glucuronide--a potent antagonist of morphine analgesia. Life Sci. 1990, 47, 579–585. [Google Scholar] [CrossRef]
- Gong, Q.L.; Hedner, T.; Hedner, J.; Bjorkman, R.; Nordberg, G. Antinociceptive and ventilatory effects of the morphine metabolites: morphine-6-glucuronide and morphine-3-glucuronide. Eur. J. Pharmacol. 1991, 193, 47–56. [Google Scholar] [CrossRef]
- Yeh, S.Y.; Gorodetzky, C.W.; Krebs, H.A. Isolation and identification of morphine 3- and 6-glucuronides, morphine 3,6-diglucuronide, morphine 3-ethereal sulfate, normorphine, and normorphine 6-glucuronide as morphine metabolites in humans. J. Pharm. Sci. 1977, 66, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Netriova, J.; Blahova, E.; Johanesova, Z.; Brandsteterova, E.; Lehotay, J.; Serdt, K.; Mocak, J. HPLC determination of morphine, morphine-3-glucuronide and morphine-6-glucuronide in human serum of oncological patients after administration of morphine drugs. Pharmazie 2006, 61, 528–534. [Google Scholar] [PubMed]
- Hartley, R.; Green, M.; Quinn, M.; Levene, M.I. Analysis of morphine and its 3- and 6-glucuronides by high performance liquid chromatography with fluorimetric detection following solid phase extraction from neonatal plasma. Biomed. Chromatogr. 1993, 7, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.W.E.; Watt, J.A.; Kennedy, M.; Cramond, T.; Smith, M.T. Quantitation of morphine, morphine-3-glucuronide, and morphine-6-glucuronide in plasma and cerebrospinal fluid using solid-phase extraction and high-performance liquid chromatography with electrochemical detection. Ther. Drug Monit. 1994, 16, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Elliott, S. A validated hybrid quadrupole linear ion-trap LC–MS method for the analysis of morphine and morphine glucuronides applied to opiate deaths. Forens. Scie. Int. 2009, 187, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, M.; Takatsuka, S.; Matsuoka, M.; Tagawa, K. Determination of morphine, morphine-3-glucuronide and morphine-6-glucuronide in monkey and dog plasma by high-performance liquid chromatography–electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2004, 35, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Gao, J.; Sun, L.; Chang, W. Determination of morphine by capillary electrophoresis immunoassay in thermally reversible hydrogel-modified buffer and laser-induced fluorescence detection. J. Chromatogr. A 2000, 895, 1–7. [Google Scholar] [CrossRef]
- Taylor, R.B.; Low, A.S.; Reid, R.G. Determination of opiates in urine by capillary electrophoresis. J. Chromatogr. B 1996, 675, 213–223. [Google Scholar] [CrossRef]
- Wey, A.B.; Thormann, W. Capillary electrophoresis-electrospray ionization ion trap mass spectrometry for analysis and confirmation testing of morphine and related compounds in urine. J. Chromatogr. A 2001, 916, 225–238. [Google Scholar] [CrossRef]
- Lurie, I.S.; Panicker, S.; Hays, P.A.; Garcıa, A.D.; Geer, B.L. Use of dynamically coated capillaries with added cyclodextrins for the analysis of opium using capillary electrophoresis. J. Chromatogr. A 2003, 984, 109–120. [Google Scholar] [CrossRef]
- Isbell, T.A.; Strickland, E.C.; Hitchcock, J.; McIntire, G.; Colyer, C.L. Capillary electrophoresis – mass spectrometry determination of morphine and its glucuronide metabolites. J. Chromatogr. B 2015, 980, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Colyer, C.L. Noncovalent labeling of proteins in capillary electrophoresis with laser-induced fluorescence detection. Cell Biochem. Biophys. 2000, 33, 323–337. [Google Scholar] [CrossRef]
- Yan, W.; Sloat, A.L.; Yagi, S.; Nakazumi, H.; Colyer, C.L. Protein labeling with red squarylium dyes for analysis by capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis 2006, 27, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Massie, T.L.; Maeda, T.; Nakazumi, H.; Colyer, C.L. On-column labeling of gram-positive bacteria with a novel boronic acid functionalized squarylium cyanine dye for analysis by polymer-enhanced capillary transient isotachophoresis. Anal. Chem. 2012, 84, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Maeda, T.; Nakazumi, H.; Colyer, C.L. An application of polymer-enhanced capillary transient isotachophoresis with an emissive boronic acid functionalized squarylium dye as an on-capillary labeling agent for gram-positive bacteria. Anal. Sci. 2013, 29, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Maahs, G.; Hegenberg, P. Syntheses and derivatives of squaric acid. Angew. Chem. Int. Ed. Engl. 1966, 5, 888–893. [Google Scholar] [CrossRef]
- Sprenger, H.E.; Ziegenbein, W. Condensation products of squaric acid and tertiary aromatic amines. Angew. Chem. Int. Ed. Engl. 1966, 6, 894. [Google Scholar] [CrossRef] [PubMed]
- Gravesteijin, D.J.; Steenbergen, C.; Vander Veen, J. Single wavelength optical recording in pure, solvent coated infrared dye layers. Proc. SPIE. Int. Soc. Opt. Eng. 1983, 420, 327–331. [Google Scholar]
- Merritt, V.Y.; Hovel, H.J. Organic solar cells of hydroxy squarylium. Appl. Phys. Lett. 1976, 29, 414–415. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.K. High performance squarylium dyes for high-tech use. Color Tech. 2001, 117, 61–67. [Google Scholar] [CrossRef]
- Welder, F.; Paul, B.; Nakazumi, H.; Yagi, S.; Colyer, C.L. Symmetric and asymmetric squarylium dyes as noncovalent protein labels: A study by fluorimetry and capillary electrophoresis. J. Chromatogr. B 2003, 793, 93–105. [Google Scholar] [CrossRef]
- Yan, W.; Colyer, C.L. Investigating noncovalent squarylium dye–protein interactions by capillary electrophoresis–frontal analysis. J. Chromatogr. A 2006, 1135, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sloat, A.L.; Roper, M.G.; Lin, X.; Ferrance, J.P.; Landers, J.P.; Colyer, C.L. Protein determination by microchip capillary electrophoresis using an asymmetric squarylium dye: Noncovalent labeling and nonequilibrium measurement of association constants. Electrophoresis 2008, 29, 3446–3455. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Rockett, S.; Massie, T.L.; Turner, G.B.; Maeda, T.; Nakazumi, H.; Colyer, C.L. Asymmetric Mono- and Bis-Squarylium Dyes as Pre-Column and On-Column Labels for Protein Analysis by Capillary Electrophoresis with Laser-induced Fluorescence Detection. J. Anal. Bioanal. Tech. 2012, S9, 1–7. [Google Scholar]
- Saito, S.; Massie, T.L.; Maeda, T.; Nakazumi, H.; Colyer, C.L. A long-wavelength fluorescent squarylium cyanine dye possessing boronic acid for sensing monosaccharides and glycoproteins with high enhancement in aqueous solution. Sensors 2012, 12, 5420–5431. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, K.; Colyer, C.L.; Sebaiy, M.; Zhou, J.; Maeda, T.; Nakazumi, H.; Shibukawa, M.; Saito, S. Molecular design of boronic acid-functionalized squarylium cyanine dyes for multiple discriminant analysis of sialic acid in biological samples: Selectivity toward monosaccharides controlled by different alkyl side chain lengths. Anal. Chem. 2015, 87, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Fan, J.; Sun, S.; Wang, L.; Song, B.; Peng, X. 1-(Carbamoylmethyl)-3H-indolium squarine dyes: Synthesis, spectra, photo-stability and association with BSA. Dyes Pigments 2010, 85, 43–50. [Google Scholar] [CrossRef]
- Tatikolov, A.S.; Costa, S.M.B. Photophysical and aggregation properties of a long-chain squarylium indocyanine dye. J. Photochem. Photobiol. A 2001, 140, 147–156. [Google Scholar] [CrossRef]
- Fukuda, K.; Nakahara, H. Spectroscopic studies on aggregation of squarylium dyes and the photopolymerization of a diacetylenic derivative in monolayers at the air/water interface. Colloid Surf. A 1995, 102, 57–68. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhao, L.M.; Zhong, D.F. A novel metabolic pathway of morphine: Formation of morphine glucosides in cancer patients. Br. J. Clin. Pharmacol. 2003, 55, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.D.; Flynn, G.L. Solubility behavior of narcotic analgesics in aqueous media: Solubilities and dissociation constants of morphine, fentanyl, and sufentanil. Pharm. Res. 1989, 6, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Colyer, C.L. Fluorimetric studies and noncovalent labeling of protein with the near-infrared dye HITCI for analysis by CE-LIF. J. Sep. Sci. 2005, 28, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; McCourtney, E.J.; Sharager, R.I. Titration studies of phytic acid. J. Amer. Oil Chem. Soc. 1982, 59, 189–191. [Google Scholar] [CrossRef]
- Okafo, G.N.; Birrell, H.C.; Greenaway, M.; Haran, M.; Camilleri, P. The effect of phytic acid on the resolution of peptides and proteins in capillary electrophoresis. Anal. Biochem. 1994, 219, 201–206. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebaiy, M.M.; El-Shanawany, A.A.; Baraka, M.M.; Abdel-Aziz, L.M.; Isbell, T.A.; Colyer, C.L. Determination of Morphine and Its Metabolites in Human Urine by Capillary Electrophoresis with Laser Induced Fluorescence Detection Employing On-Column Labeling with a New Boronic Acid Functionalized Squarylium Cyanine Dye. Separations 2016, 3, 1. https://doi.org/10.3390/chromatography3010001
Sebaiy MM, El-Shanawany AA, Baraka MM, Abdel-Aziz LM, Isbell TA, Colyer CL. Determination of Morphine and Its Metabolites in Human Urine by Capillary Electrophoresis with Laser Induced Fluorescence Detection Employing On-Column Labeling with a New Boronic Acid Functionalized Squarylium Cyanine Dye. Separations. 2016; 3(1):1. https://doi.org/10.3390/chromatography3010001
Chicago/Turabian StyleSebaiy, Mahmoud M., Abdullah A. El-Shanawany, Mohamed M. Baraka, Lobna M. Abdel-Aziz, Theresa A. Isbell, and Christa L. Colyer. 2016. "Determination of Morphine and Its Metabolites in Human Urine by Capillary Electrophoresis with Laser Induced Fluorescence Detection Employing On-Column Labeling with a New Boronic Acid Functionalized Squarylium Cyanine Dye" Separations 3, no. 1: 1. https://doi.org/10.3390/chromatography3010001







