Recent Advances in Nanomaterial-Based Sensing for Food Safety Analysis
Abstract
:1. Introduction
2. Current Approaches for Food Safety Sensing
3. Optical Sensors and Biosensors
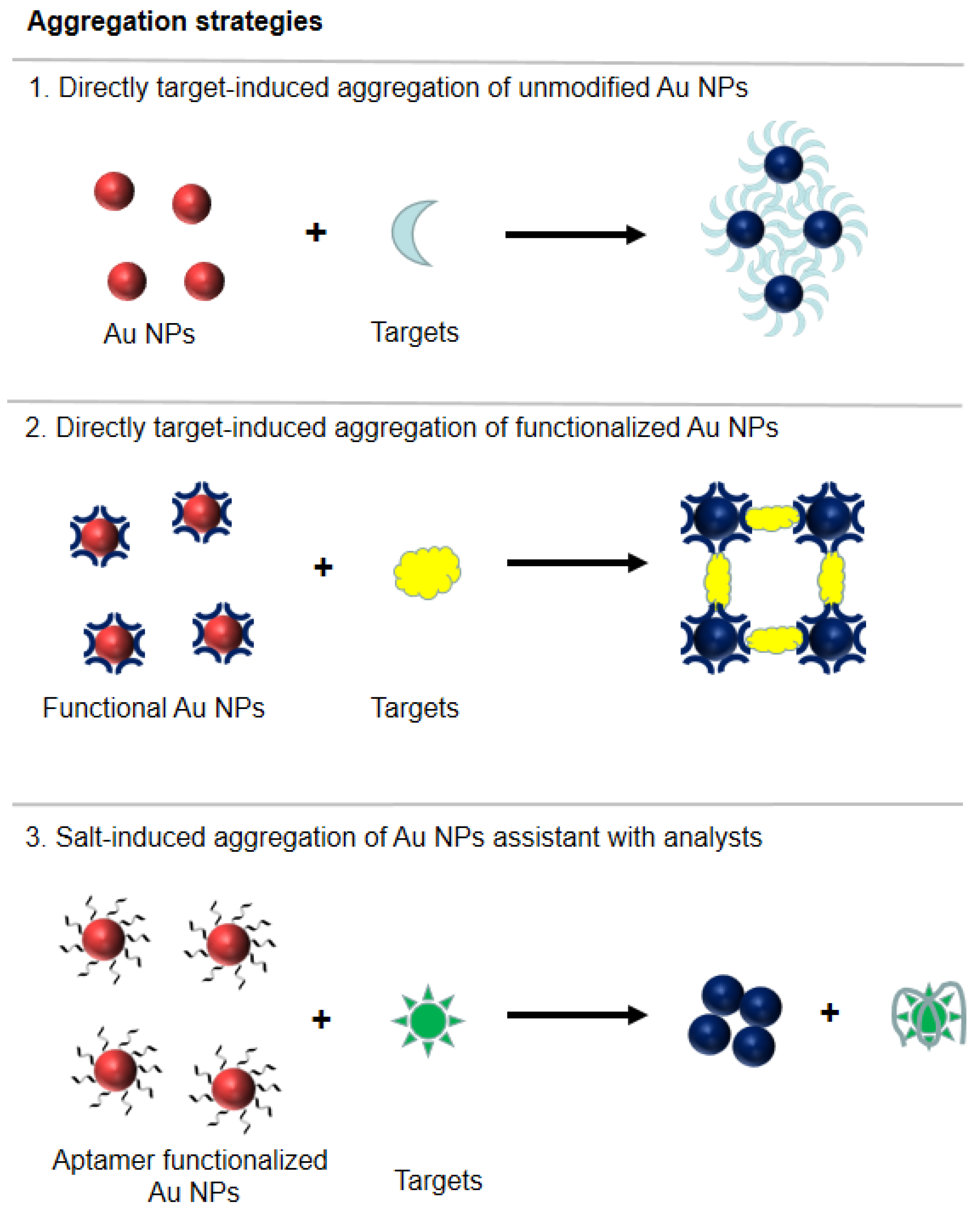
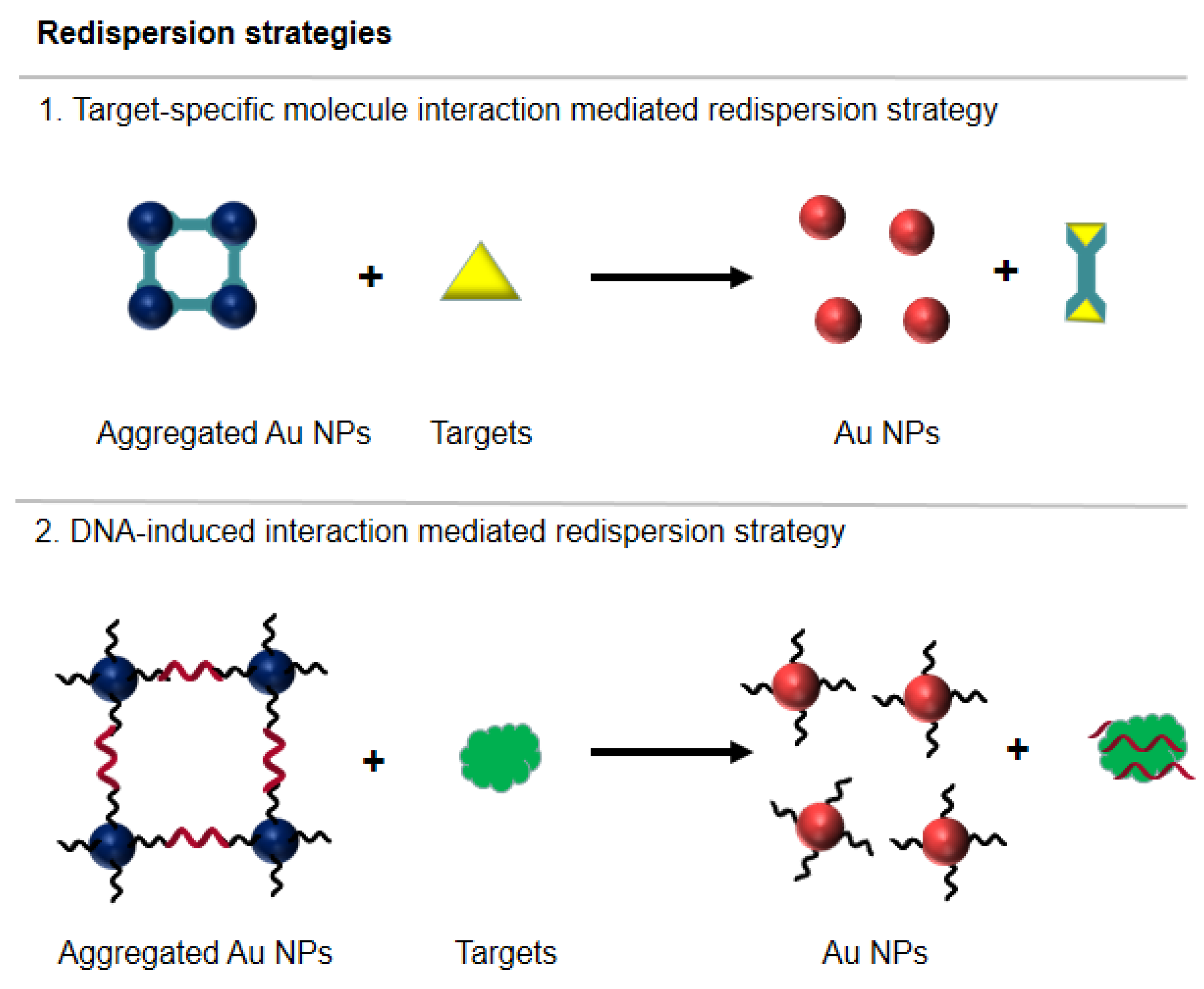
3.1. Colorimetric Sensors and Biosensors
3.2. Fluorescence Sensors and Biosensors
3.3. Surface Plasmon Resonance (SPR) Sensors and Biosensors
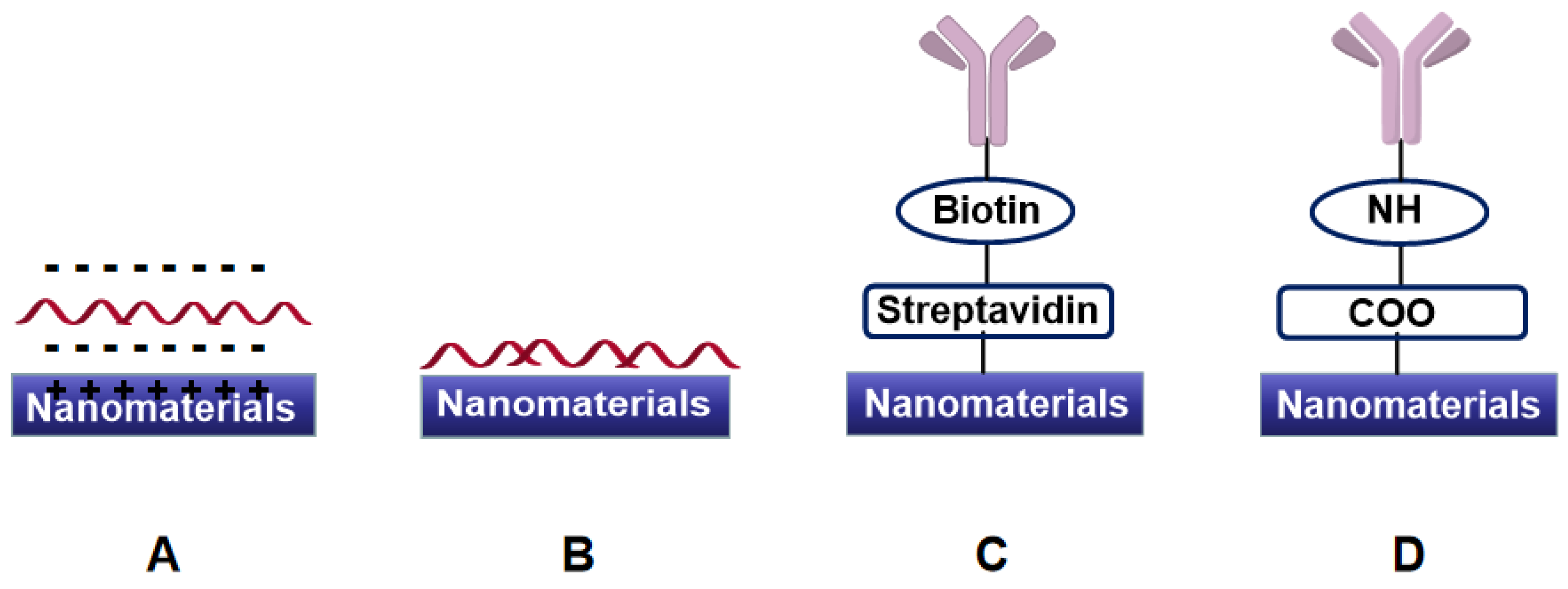
4. Electrochemical Sensors and Biosensors
4.1. Impedance Sensors and Biosensors
4.2. Voltammetry Sensors and Biosensors
4.3. Potentiometric Sensors and Biosensors
5. Mobile Sensors and Biosensors
5.1. Smartphone-Based Optical Biosensors
5.2. Smartphone-Based Electrochemical Biosensors
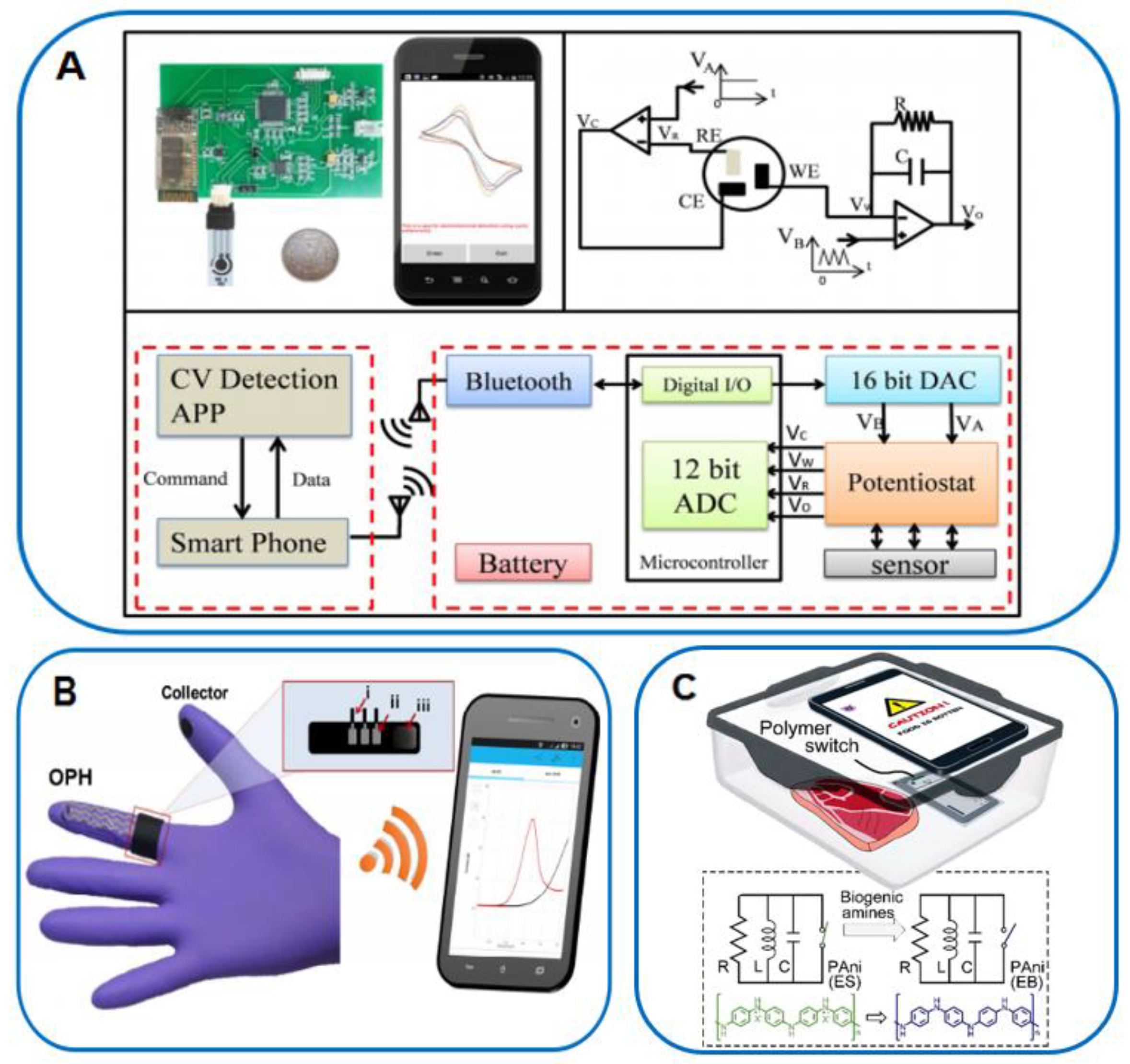
6. Conclusion and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Boyaci, I.H.; Temiz, H.T.; Genis, H.E.; Soykut, E.A.; Nur Yazgan, N.N.; Güven, B.; Uysal, R.S.; Bozkurt, A.G.; İlaslan, K.; Torun, O.; et al. Dispersive and FT-Raman spectroscopic methods in food analysis. RSC Adv. 2015, 5, 56606–56624. [Google Scholar] [CrossRef]
- Li, G.L.; Fu, Y.H.; Han, X.S.; Li, X.Y.; Li, C.C. Metabolomic investigation of porcine muscle and fatty tissue after Clenbuterol treatment using gas chromatography/mass spectrometry. J. Chromatogr. A 2016, 1456, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xu, F.; Jiang, H.Y.; Hou, Y.L.; Rao, Q.X.; Guo, P.J.; Ding, S.Y. A novel quantum dot-based fluoroimmunoassay method for detection of Enrofloxacin residue in chicken muscle tissue. Food Chem. 2009, 113, 1197–1201. [Google Scholar] [CrossRef]
- Wu, Y.N.; Zhao, Y.F.; Li, J.G. A Survey on Occurrence of Melamine and Its Analogues in Tainted Infant Formula in China. Biomed. Environ. Sci. 2009, 22, 95–99. [Google Scholar] [CrossRef] [PubMed]
- García-Cañas, V.; Simó, C.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Present and future challenges in food analysis: Foodomics. Anal. Chem. 2012, 84, 10150–10159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, F.; Volpe, G.; Micheli, L.; Palleschi, G. A review on novel developments and applications of immunosensors in food analysis. Anal. Chim. Acta 2007, 605, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V.; Arduini, F.; Palleschi, G.; Rea, G. Biosensing technology for sustainable food safety. TrAC Trends Anal. Chem. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Amine, A.; Mohammadi, H.; Bourais, I.; Palleschi, G. Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens. Bioelectron. 2006, 21, 1405–1423. [Google Scholar] [CrossRef] [PubMed]
- Campàs, M.; Garibo, D.; Prieto-Simon, B. Novelano biotechnological concepts in electrochemical biosensors for the analysis of toxins. Analyst 2012, 137, 1055–1067. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfareanalyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef]
- Sharma, R.; Ragavan, K.V.; Thakur, M.S.; Raghavarao, K.S.M.S. Recent advances in nanoparticle based aptasensors for food contaminants. Biosens. Bioelectron. 2015, 74, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Yaqub, M.; Rahim, A.; Catanante, G.; Hayat, A.; Marty, J.L. An overview on recent progress inelectrochemical biosensors for antimicrobial drug residues in animal-derived food. Sensors 2017, 17, 1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhukhovitskiy, A.V.; MacLeod, M.J.; Johnson, J.A. Carbene Ligands in Surface Chemistry: From Stabilization of Discrete Elemental Allotropes to Modification of Nanoscale and Bulk Substrates. Chem. Rev. 2015, 115, 11503–11532. [Google Scholar] [CrossRef]
- Wang, G.Q.; Rühling, A.; Amirjalayer, S.; Marek, K.; Ernst, J.B.; Richter, C.; Gao, H.J.; Timmer, A.; Gao, H.Y.; Doltsinis, N.L.; et al. Ballbot-type motion of N-heterocyclic carbenes on gold surfaces. Nat. Chem. 2017, 9, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Misztalewska-Turkowicz, I.; Markiewicz, K.H.; Michalak, M.; Wilczewska, A.Z. NHC-copper complexes immobilized on magnetic nanoparticles: Synthesis and catalytic activity in the CuAAC reactions. J. Catal. 2018, 362, 46–54. [Google Scholar] [CrossRef]
- Engel, S.; Fritz, E.C.; Ravoo, B.J. New trends in the functionalization of metallic gold: From organosulfur ligands to N-heterocyclic carbenes. Chem. Soc. Rev. 2017, 46, 2057–2075. [Google Scholar] [CrossRef]
- Bernardos, M.D.D.L.; Pérez-Rodríguez, S.; Gual, A.; Claver, C.; Godard, C. Facile synthesis of NHC-stabilized Ni nanoparticles and their catalytic application in the Z-selective hydrogenation of alkynes. Chem. Commun. 2017, 53, 7894–7897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Langille, M.R.; Mirkin, C.A. Synthesis of Silver Nanorods by Low Energy Excitation of Spherical Plasmonic Seeds. Nano Lett. 2011, 11, 2495–2498. [Google Scholar] [CrossRef]
- Yu, X.F.; Liu, J.W.; Cong, H.P.; Xue, L.; Arsad, M.N.; Albar, H.A.; Sobahi, T.R.; Gao, Q.; Yu, S.H. Template- and surfactant-free synthesis of ultrathin CeO2 nanowires in a mixed solvent and their superior adsorption capability for water treatment. Chem. Sci. 2015, 6, 2511–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanal, B.P.; Zubarev, E.R. Chemical Transformation of Nanorods to Nanowires: Reversible Growth and Dissolution of Anisotropic Gold Nanostructures. ACS Nano 2019, 13, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Sun, W.S.; Liang, X.; Zou, H.Y.; Jiao, X.; Lin, K.A.; Li, T.L. Two-dimensional Fe2O3 nanosheets as adsorbent for the removal of Pb(II) from aqueous solution. J. Mol. Liq. 2021, 335, 116197. [Google Scholar] [CrossRef]
- Zhang, S.J.; Pelligra, C.I.; Feng, X.D.; Osuji, C.O. Directed assembly of hybrid nanomaterials and nanocomposites. Adv. Mater. 2018, 30, 1705794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Zhou, H.; Chen, W.X.; Tong, Y.J.; Zhao, C.; Lin, Y.; Jiang, Z.; Zhang, Q.W.; Xue, Z.G.; Cheong, W.C.; et al. Two-step carbothermal welding to access atomically dispersed Pd1 on three-dimensional zirconia nanonet for direct indole synthesis. J. Am. Chem. Soc. 2019, 141, 10590–10594. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.N.; Huang, R.L.; Qi, W.; Shi, M.B.; Su, R.X.; He, Z.M. Controllable synthesis of ZnO nanoflowers with structure-dependent photocatalytic activity. Catal. Today 2020, 355, 397–407. [Google Scholar] [CrossRef]
- Argentero, G.; Mittelberger, A.; Monazam, M.R.A.; Cao, Y.; Pennycook, T.J.; Mangler, C.; Kramberger, C.; Kotakoski, J.; Geim, A.K.; Meyer, J.C. Unraveling the 3D atomic structure of a suspended graphene/hBN van der Waals heterostructure. Nano Lett. 2017, 17, 1409–1416. [Google Scholar] [CrossRef] [Green Version]
- Ling, X.; Roland, S.; Pileni, M.P. Supracrystals of N-Heterocyclic Carbene-Coated Au Nanocrystals. Chem. Mater. 2015, 27, 414–423. [Google Scholar] [CrossRef]
- Roland, S.; Ling, X.; Pileni, M.P. N-Heterocyclic Carbene Ligands for Au Nanocrystal Stabilization and Three-Dimensional Self-Assembly. Langmuir 2016, 32, 7683–7696. [Google Scholar] [CrossRef] [Green Version]
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Liu, B.W.; Liu, J.W. Sensors and biosensors based on metal oxide nanomaterials. TrAC 2019, 121, 115690. [Google Scholar] [CrossRef]
- Kwon, S.O.; Song, H.S.; Park, H.T.; Jang, J. Conducting Nanomaterial Sensor Using Natural Receptors. Chem. Rev. 2019, 119, 36–93. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Huang, N.M.; Zainal, Z.; Ahmad, S.A.A. Significance of nanomaterials in electrochemical sensors for nitrate detection: A review. Trends Environ. Anal. Chem. 2021, 31, e00135. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2017, 103, 113–129. [Google Scholar] [CrossRef]
- Holzinger, M.; Goff, A.L.; Cosnier, S. Synergetic effects of combined nano_materials for biosensing applications. Sensors 2017, 17, 1010. [Google Scholar] [CrossRef] [Green Version]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Siva, S.; Jin, J.O.; Choi, I.; Kim, M. Nanoliposome based biosensors for probing mycotoxins and their applications for food: A review. Biosens. Bioelectron. 2022, 219, 114845. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Guo, S.J. Chemically doped fluorescent carbon and graphene quantum dots for bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef]
- Pirsa, S.; Heidari, H.; Lotfi, J. Design selective gas sensors based on nano-sized polypyrrole/polytetrafluoroethylene and polypropylene membranes. IEEE Sens. J. 2016, 16, 2922–2928. [Google Scholar] [CrossRef]
- Arshad, F.; Mohd-Naim, N.F.; Chandrawati, R.; Cozzolino, D.; Ahmed, M.U. Nanozyme-based sensors for detection of food biomarkers: A review. RSC Adv. 2022, 12, 26160–26175. [Google Scholar] [CrossRef]
- Lan, L.Y.; Yao, Y.; Ping, J.F.; Ying, Y.B. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Yang, G.H.; Li, H.; Du, D.; Lin, Y.H. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2016, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Zhao, J.L.; Liang, L.J. Recent development of antibiotic detection in food and environment: The combination of sensors and nanomaterials. Microchim. Acta 2021, 188, 21. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.J.; Sharma, P.; Bora, U. Recent developments in application of nucleic acid aptamer in food safety. Food Control 2022, 145, 109406. [Google Scholar] [CrossRef]
- Xing, C.R.; Jing, X.X.; Zhang, X.; Yuan, J. Ultrasensitive indirect competitive ELISA and strip sensor for detection of furazolidone metabolite in animal tissues. Food Agric. Immunol. 2017, 28, 1269–1282. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Liu, L.Q.; Xu, L.G.; Song, S.S.; Kuang, H.; Cui, G.; Xu, C.L. Gold nanoparticle-based paper sensor for ultrasensitive and multiple detection of 32 (fluoro)quinolones by one monoclonal antibody. Nano Res. 2017, 10, 108–120. [Google Scholar] [CrossRef]
- Sani, N.D.; Heng, L.Y.; Marugan, R.S.P.M.; Rajab, N.F. Electrochemical DNA biosensor for potential carcinogen detection in food sample. Food Chem. 2018, 296, 503–510. [Google Scholar] [CrossRef]
- Suaifan, G.A.R.Y.; Alhogail, S.; Zourob, M. Paper-based magnetic nanoparticle-peptide probe for rapid and quantitative colorimetric detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2017, 92, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.T.; Zhang, T.Y.; Li, Z.; Liu, X.N.; Zhu, Y.C.; Zhao, W.W.; Chen, H.Y.; Xu, J.J. Photoelectrochemical Cytosensors. Electroanalysis 2022, 34, 947–955. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Santos, T.A.P.R. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Hasseb, A.A.; Ghani, N.T.A.; Shehab, O.R.; Nashar, R.M. Application of molecularly imprinted polymers for electrochemical detection of some important biomedical markers and pathogens. Curr. Opin. Electrochem. 2022, 31, 100848. [Google Scholar] [CrossRef]
- Morales, M.A.; Halpern, J.M. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjug. Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Goff, A.L.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 2296–2646. [Google Scholar] [CrossRef] [Green Version]
- Putzbach, W.; Ronkainen, N.J. Immobilization Techniques in the Fabrication of Nanomaterial-Based Electrochemical Biosensors: A Review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Zhang, L.; Hu, Y.; Zhou, C.S.; Lan, W.; Fu, H.Y.; She, Y.B. Nanomaterials as optical sensors for application in rapid detection of food contaminants, quality and authenticity. Sens. Actuators B Chem. 2021, 329, 129135. [Google Scholar] [CrossRef]
- Fang, L.; Jia, M.X.; Zhao, H.P.; Kang, L.Z.; Shi, L.C.; Zhou, L.D.; Kong, W.J. Molecularly imprinted polymer-based optical sensors for pesticides in foods: Recent advances and future trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Yoo, S.M.; Lee, S.Y. Optical biosensors for the detection of pathogenic microorganisms. Trends Biotechnol. 2016, 34, 7–25. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.B.; Chen, X.Y. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhou, K.; Zhao, G.H. Gold nanoparticles: From synthesis, properties to their potential application as colori-metric sensors in food safety screening. Trends Food Sci. Technol. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Liu, G.Y.; Lu, M.; Huang, X.D.; Li, T.F.; Xu, D.H. Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening. Sensors 2018, 18, 4166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Salmain, M.; Liedberg, B.; Boujday, S. Naked Eye Immunosensing of Food Biotoxins Using Gold Nanoparticle-Antibody Bioconjugates. ACS Appl. Nano Mater. 2019, 2, 4150–4158. [Google Scholar] [CrossRef]
- Li, H.; Gan, J.C.; Yang, Q.; Fu, L.L.; Wang, Y.B. Colorimetric detection of food freshness based on amine-responsive dopamine polymerization on gold nanoparticles. Talanta 2021, 234, 122706. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nano tube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Jiang, M.Y.; Niu, N.; Chen, Z.J.; Li, S.J.; Liu, S.X.; Li, J. Natural-product derived carbon dots: From natural products to functional materials. ChemSusChem 2018, 11, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.N.; Zhu, C.N.; Gu, R.R.; Hu, J.; Xu, Y.; Ye, S.; Zhu, J. Copper-Modified Double-Emission Carbon Dots for Rapid Detection of Thiophanate Methyl in Food. Foods 2022, 11, 3336. [Google Scholar] [CrossRef]
- Yin, Q.H.; Wang, M.T.; Fang, D.; Zhu, Y.Q.; Yang, L.H. Novel N,Cl-doped deep eutectic solvents-based carbon dots as a selective fluorescent probe for determination of morphine in food. RSC Adv. 2021, 11, 16805–16813. [Google Scholar] [CrossRef]
- Hu, Q.; Gong, X.J.; Liu, L.Z.; Choi, M.M.F. Characterization and analytical separation of fluorescent carbon nanodots. J. Nanomater. 2017, 2017, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.K.; Ha, S.K.; Verma, K.; Tiwari, S.K. Recent progress in selected bionanomaterials and their engineering applications: An overview. J. Sci. Adv. Mater. Dev. 2018, 3, 263–288. [Google Scholar] [CrossRef]
- Safarik, I.; Baldikova, E.; Prochazkova, J.; Safarikova, M.; Pospiskova, K. Magnetically modified agricultural and food waste: Preparation and application. J. Agric. Food Chem. 2018, 66, 2538–2552. [Google Scholar] [CrossRef]
- Qu, J.H.; Wei, Q.Y.; Sun, D.W. Carbon dots: Principles and their applications in food quality and safety detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef]
- Xue, L.; Liu, G.; Parfitt, J.; Liu, X.J.; Herpen, E.V.; Stenmarck, Å.; O’Connor, C.; Östergren, K.; Cheng, S.K. Missingfood, missing data? A critical review of global food losses and food waste data. Environ. Sci. Technol. 2017, 51, 6618–6633. [Google Scholar] [CrossRef] [PubMed]
- Grainger, M.J.; Aramyan, L.; Logatcheva, K.; Piras, S.; Righi, S.; Setti, M.; Vittuari, M.; Stewart, G.B. The use of systems models to identify food waste drivers. Glob. Food Secur. 2018, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Menna, F.D.; Dietershagen, J.; Loubiere, M.; Vittuari, M. Life cycle costing of food waste: A review of methodological approaches. Waste Manag. 2018, 73, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Y.T.; Guo, T.T.; Yang, T.; Chen, M.L.; Wang, J.H. Green preparation of carbon dots with papaya as carbon source for effective fluorescent sensing of Iron (III) and Escherichia coli. Biosens. Bioelectron. 2016, 85, 68–75. [Google Scholar] [CrossRef]
- Das, P.; Bose, M.; Ganguly, S.; Mondal, S.; Das, A.K.; Banerjee, S.; Das, N.C. Green approach to photoluminescent carbon dots for imaging of gram-negative bacteria Escherichia coli. Nanotechnology 2017, 28, 195501–195513. [Google Scholar] [CrossRef]
- Hu, X.T.; Li, Y.X.; Xu, Y.W.; Gan, Z.Y.; Zou, X.B.; Shi, J.Y.; Huang, X.W.; Li, Z.H.; Li, Y.H. Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk. Food Chem. 2021, 339, 127775. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, C.; Sun, X.B.; Pan, W.; Yu, G.G.; Wang, J.P. Highly crystalline carbon dots from fresh tomato: UV emission and quantum confinement. Nanotechnology 2017, 28, 485705. [Google Scholar] [CrossRef]
- Bao, R.Q.; Chen, Z.Y.; Zhao, Z.W.; Sun, X.; Zhang, J.Y.; Hou, L.R.; Yuan, C.Z. Green and facile synthesis of nitrogen and phosphorus co-doped carbon quantum dots towards fluorescent ink and sensing applications. Nanomaterials 2018, 8, 386. [Google Scholar] [CrossRef] [Green Version]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Shen, J.; Shang, S.M.; Chen, X.J.; Wang, D.; Cai, Y. Facile synthesis of fluorescence carbon dots from sweet potato for Fe3+ sensing and cell imaging. Mater. Sci. Eng. C 2017, 76, 856–864. [Google Scholar] [CrossRef]
- Zhao, J.J.; Huang, M.J.; Zhang, L.L.; Zou, M.B.; Chen, D.X.; Huang, Y.; Zhao, S.L. Unique ap proach to develop carbon dot-based nanohybrid near-infrared ratiometric fluorescent sensor for the detection of mercury ions. Anal Chem. 2017, 89, 8044–8049. [Google Scholar] [CrossRef] [PubMed]
- Bano, D.; Kumar, V.; Singh, V.K.; Hasan, S.H. Green synthesis of fluorescent carbon quantum dots for the detection of mercury(ii) and glutathione. New J. Chem. 2018, 42, 5814–5821. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sen sing of Pb2+ ions and live cell imaging. Sens. Actuators B Chem. 2017, 242, 679–686. [Google Scholar] [CrossRef]
- Bhatt, S.; Bhatt, M.; Kumar, A.; Vyas, G.; Gajaria, T.; Paul, P. Green route for synthesis of multifunctional fluorescent carbon dots from Tulsi leaves and its application as Cr(VI) sensors, bio-imaging and patterning agents. Colloids Surf. B Biointerfaces 2018, 167, 126–133. [Google Scholar] [CrossRef]
- Wen, X.P.; Shi, L.H.; Wen, G.G.; Li, Y.Y.; Dong, C.; Yang, J.; Shuang, S.M. Green and facile synthesis of nitrogen-doped carbon nanodots for multicolor cellular imaging and Co2+ sensing in living cells. Sens. Actuators B Chem. 2016, 235, 179–187. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhou, Q.X.; Li, J.; Lei, M.; Yan, X.Y. Selective and sensitive chemosensor for lead ions using fluorescent carbon dots prepared from chocolate by one-step hydro thermal method. Sens. Actuators B Chem. 2016, 237, 597–604. [Google Scholar] [CrossRef]
- Cai, Y.B.; Li, L.Z.; Wang, Z.T.; Sun, J.Z.; Qin, A.J.; Tang, B.Z. A sensitivity tuneable tetraphenylethene-based fluorescent probe for directly indicating the concentration of hydrogen sulfide. Chem. Commun. 2014, 50, 8892–8895. [Google Scholar] [CrossRef]
- Bu, F.; Wang, E.J.; Peng, Q.; Hu, R.R.; Qin, A.J.; Zhao, Z.J.; Tang, B.Z. Structural and theoretical insights into the AIE attributes of phosphindole oxide: The balance between rigidity and flexibility. Chem. Eur. J. 2015, 21, 4440–4449. [Google Scholar] [CrossRef]
- Jing, H.; Lu, L.; Feng, Y.; Zheng, J.F.; Deng, L.D.; Chen, E.Q.; Ren, X.K. Synthesis, Aggregation-Induced Emission, and Liquid Crystalline Structure of Tetraphenylethylene–Surfactant Complex via Ionic Self-Assembly. J. Phys. Chem. C. 2016, 120, 27577–27586. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Leung, N.L.C.; Zhang, J.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE-based luminescence probes for metal ion detection. Coord. Chem. Rev. 2020, 429, 213693. [Google Scholar] [CrossRef]
- Wang, D.; Tang, B.Z. Acc. Aggregation-Induced Emission Luminogens for Activity-Based Sensing. Chem. Res. 2019, 52, 2559–2570. [Google Scholar] [CrossRef]
- Jiang, M.J.; Gu, X.G.; Lam, J.W.Y.; Zhang, Y.L.; Kwok, R.T.K.; Wong, K.S.; Tang, B.Z. Two-photon AIE bio-probe with large Stokes shift for specific imaging of lipid droplets. Chem. Sci. 2017, 8, 5440–5446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, J.X.; Li, H.X.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular materials based on AIE luminogens (AIEgens): Construction and applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. AIE-based cancer theranostics. Coordin. Chem. Rev. 2020, 402, 213076. [Google Scholar] [CrossRef]
- Hong, Y.; Meng, L.; Chen, S.; Leung, C.W.; Da, L.T.; Faisal, M.; Silva, D.A.; Liu, J.Z.; Lam, J.W.Y.; Huang, X.H.; et al. Monitoring and inhibition of insulin fibrillation by a small organic fluorogen with aggregation-induced emission characteristics. J. Am. Chem. Soc. 2012, 134, 1680–1689. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Lam, W.Y.; Tang, B.Z. Tetraphenylethene: A versatile AIE building block for the construction of efficient luminescent materials for organic light-emitting diodes. J. Mater. Chem. 2012, 22, 23726–23740. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, Y.; Liang, H.; Ho, C.L.; Zhao, Q.; Huang, W.; Wong, W.Y. A water-soluble tetraphenylethene based probe for luminescent carbon dioxide detection and its biological application. J. Mater. Chem. C 2015, 3, 11850–11856. [Google Scholar] [CrossRef]
- Hu, R.R.; Leung, N.L.C.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Lam, J.W.Y.; Hong, Y.; Faisal, M.; Yuan, W.Z.; Tang, B.Z. Simple biosensor with high selectivity and sensitivity: Thiol-specific biomolecular probing and intracellular imaging by AIE fluorogen on a TLC plate through a thiol-ene click mechanism. Chem. Eur. J. 2010, 16, 8433–8438. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.N.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Jia, Y.M.; Wu, F.; Liu, P.L.; Zhou, G.H.; Yu, B.; Lou, X.D.; Xia, F. A label-free fluorescent aptasensor for the detection of Aflatoxin B1 in food samples using AIEgens and graphene oxide. Talanta 2019, 198, 71–77. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.L.; Wang, B.L.; Li, D.D.; Yu, J.H. Fluorescent sensors based on AIEgen-functionalised mesoporous silica nanoparticles for the detection of explosives and antibiotics. Inorg. Chem. Front. 2018, 5, 2183–2188. [Google Scholar] [CrossRef]
- Mehta, P.K.; Neupane, L.N.; Park, S.H.; Lee, K.H. Ratiometric fluorescent detection of silver nanoparticles in aqueous samples using peptide-based fluorogenic probes with aggregation-induced emission characteristics. J. Hazard. Mater. 2021, 411, 125041. [Google Scholar] [CrossRef]
- Han, A.; Xiong, L.; Hao, S.J.; Yang, Y.Y.; Li, X.; Fang, G.Z.; Liu, J.F.; Pei, Y.; Wang, S. Highly bright self-assembled copper nanoclusters: A novel photoluminescent probe for sensitive detection of histamine. Anal. Chem. 2018, 90, 9060–9067. [Google Scholar] [CrossRef]
- McDonnell, J.M. Surface plasmon resonance: Towards an understanding of the mechanisms of biological molecular recognition. Curr. Opin. Chem. Biol. 2001, 5, 572–577. [Google Scholar] [CrossRef]
- Boozer, C.; Kim, G.; Cong, S.X.; Guan, H.; Londergan, T. Looking towards label-free biomolecular interaction analysis in a high-throughput format: A review of new surface plasmon resonance technologies. Curr. Opin. Biotechnol. 2006, 17, 400–405. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pandey, A.K.; Kaur, B. A Review of advancements (2007–2017) in plasmonics-based optical fiber sensors. Opt. Fiber Technol. 2018, 43, 20–34. [Google Scholar] [CrossRef]
- Adegoke, O.; Morita, M.; Kato, T.; Ito, M.; Suzuki, T.; Park, E.Y. Localized surface plasmon resonance-mediated fluorescence signals in plasmonic nanoparticle-quantum dot hybrids for ultrasensitive Zika virus RNA detection via hairpin hybridization assays. Biosens. Bioelectron. 2017, 94, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Lertvachirapaiboon, C.; Baba, A.; Ekgasit, S.; Shinbo, K.; Kato, K.; Kaneko, F. Transmission surface plasmon resonance techniques and their potential biosensor applications. Biosens. Bioelectron. 2018, 99, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherová-Lísalová, H.; Víšová, I.; Ermini, M.L.; Špringer, T.; Song, X.C.; Mrázek, J.; Lamačová, J.; Lynn, N.S.; Šedivák, P.; Homola, J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Écija-Arenas, A.; Kirchner, E.M.; Hirsch, T.; Fernández-Romero, J.M. Development of an aptamer-based SPR-biosensor for the determination of kanamycin residues in foods. Anal. Chim. Acta 2021, 1169, 338631. [Google Scholar] [CrossRef]
- Tan, A.; Lim, C.; Zou, S.; Ma, Q.; Gao, Z.Q. Electrochemical nucleic acid biosensors: From fabrication to application. Anal. Methods 2016, 8, 5169–5189. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Finale, C.; Scarponi, G. Square-wave anodic-stripping voltammetric determination of Cd, Pb and Cu in wine: Set-up and optimization of sample pre-treatment and instru mental parameters. Electrochim. Acta 2013, 104, 148–161. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Ahmadi, F.; Fakhari, F. Voltammetric determination of Pb, Cd, Zn, Cu and Se in milk and dairy products collected from Iran: An emphasis on permissible limits and risk assessment of exposure to heavy metals. Food Chem. 2016, 192, 1060–1067. [Google Scholar] [CrossRef]
- Belkhamssa, N.; Justino, C.I.; Santos, P.S.; Cardoso, S.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T.; Ksibi, M. Label-free disposable immunosensor for detection of atrazine. Talanta 2016, 146, 430–434. [Google Scholar] [CrossRef] [Green Version]
- Fayemi, O.E.; Adekunle, A.S.; Ebenso, E.E. A sensor for the determination of lindane using PANI/Zn, Fe (III) oxides and nylon 6, 6/MWCNT/Zn, Fe (III) oxides nanofibers modified glassy carbon electrode. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Wong, A.; Foguel, M.V.; Khan, S.; de Oliveira, F.M.; Tarley, C.R.T. Sotomayor MD. Development of an electrochemical sensor modified with MWCNT-COOH and MIP for detection of diuron. Electrochim. Acta 2015, 182, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.; Scontri, M.; Materon, E.M.; Lanza, M.R.; Sotomayor, M.D. Development and application of an electrochemical sensor modified with multi-walled carbon nanotubes and graphene oxide for the sensitive and selective detection of tetracycline. J. Electroanal. Chem. 2015, 757, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Cámara-Martos, F.; da Costa, J.; Justino, C.I.; Cardoso, S.; Duarte, A.C.; Rocha-Santos, T. Disposable biosensor for detection of iron (III) in wines. Talanta 2016, 154, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Mayorga-Martinez, C.C.; Quesada-González, D.; Zamora-Gálvez, A.; de la Escosura-Muñiz, A.; Merkoçi, A. Label-free impedimetric aptasensor for ochratoxin-A detection using iridium oxide nanoparticles. Anal Chem. 2015, 87, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, C.; Muthupandi, K.; Chen, S.M.; Ali, M.A.; Palanisamy, S.; Rajan, A.; Prakash, P.; Al-Hemaid, F.M.A.; Lou, B.S. Green synthesized silver nanoparticles decorated on reduced graphene oxide for enhanced electrochemical sensing of nitrobenzene in waste water samples. RSC Adv. 2015, 5, 31139–31146. [Google Scholar] [CrossRef]
- Niu, P.; Fernández-Sánchez, C.; Gich, M.; Ayora, C.; Roig, A. Electroanalytical assessment of heavy metals in waters with bismuth nanoparticle-porous carbon paste electrodes. Electrochim. Acta 2015, 165, 155–161. [Google Scholar] [CrossRef]
- Yu, R.Z.; Wang, R.; Wang, Z.Y.; Zhu, Q.S.; Dai, Z.H. Applications of DNA-nanozyme-based sensors. Analyst 2021, 146, 1127–1141. [Google Scholar] [CrossRef]
- He, Y.Q.; Gao, Y.; Gu, H.W.; Meng, X.Z.; Yi, H.C.; Chen, Y.; Sun, W.Y. Target-induced activation of DNAzyme for sensitive detection of bleomycin by using a simple MOF-modified electrode. Biosens. Bioelectron. 2021, 178, 113034. [Google Scholar] [CrossRef]
- Chai, C.H.; Oh, S.W. Electrochemical impedimetric biosensors for food safety. Food Sci. Biotechnol. 2020, 29, 879–887. [Google Scholar] [CrossRef]
- Mejri-Omrani, N.; Miodek, A.; Zribi, B.; Marrakchi, M.; Hamdi, M.; Marty, J.L.; Korri-Youssoufi, H. Direct detection of OTA by impedimetric aptasensor based on modified polypyrrole-dendrimers. Anal. Chim. Acta 2016, 920, 37–46. [Google Scholar] [CrossRef]
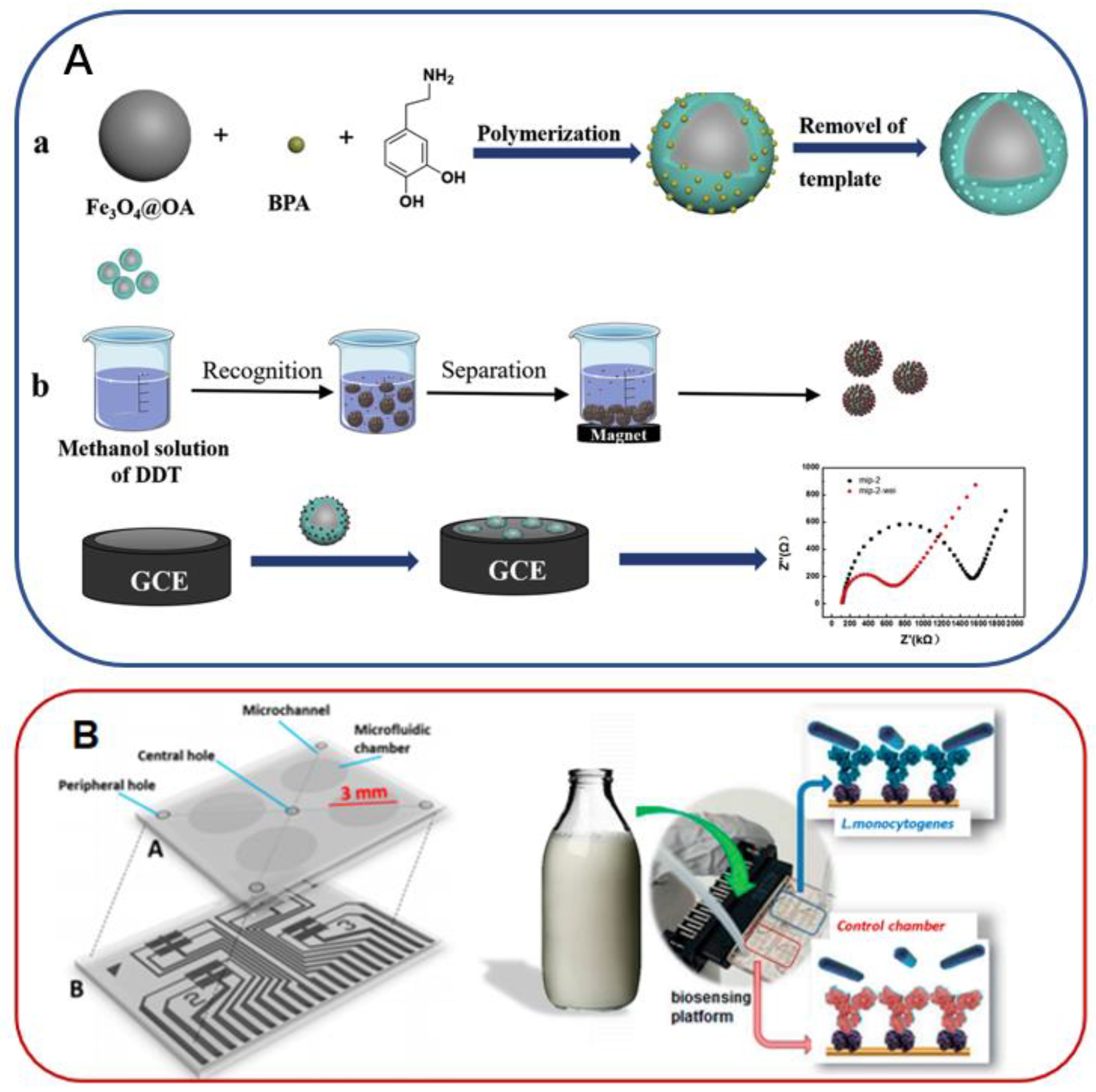
- Miao, J.N.; Liu, A.R.; Wu, L.N.; Yu, M.Z.; Wei, W.; Liu, S.Q. Magnetic ferroferric oxide and polydopamine molecularly imprinted polymer nanocomposites based electrochemical impedance sensor for the selective separation and sensitive determination of dichlorodiphenyltrichloroethane (DDT). Anal. Chim. Acta 2020, 1095, 82–92. [Google Scholar] [CrossRef]
- Chiriacò, M.; Parlangeli, I.; Sirsi, F.; Poltronieri, P.; Primiceri, E. Impedance Sensing Platform for Detection of the Food Pathogen Listeria monocytogenes. Electronics 2018, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Somerson, J.; Plaxco, K. Electrochemical aptamer-based sensors for rapid point-of-use monitoring of the mycotoxin ochratoxin a directly in a food stream. Molecules 2018, 23, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezhilan, M.; Gumpu, M.B.; Ramachandra, B.L.; Nesakumar, N.; Babu, K.J.; Krishnan, U.M.; Rayappan, J.B.B. Design and development of electrochemical biosensor for the simultaneous detection of melamine and urea in adulterated milk samples. Sens. Actuators B Chem. 2017, 238, 1283–1292. [Google Scholar] [CrossRef]
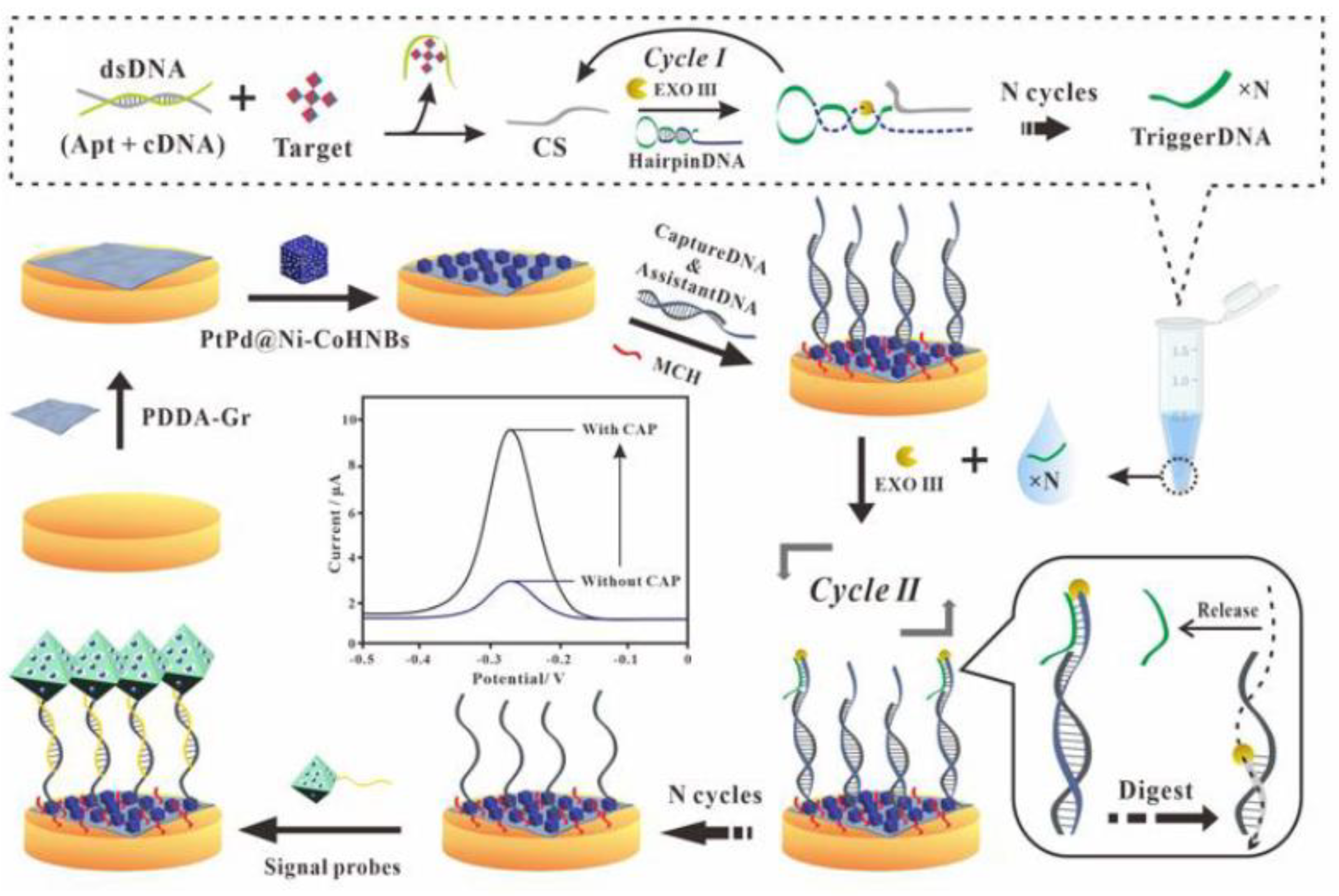
- Wang, S.Y.; He, B.S.; Liang, Y.; Jin, H.L.; Wei, M.; Ren, W.J.; Suo, Z.G.; Wang, J.S. Exonuclease III-Driven Dual-Amplified Electrochemical Aptasensor Based on PDDA-Gr/PtPd@Ni-Co Hollow Nanoboxes for Chloramphenicol Detection. ACS Appl. Mater. Interfaces 2021, 13, 26362–26372. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.L.; Hou, J.Z.; Zhao, Y.N.; Bao, J.; Yang, M.; Fa, H.B.; Yang, Y.X.; Li, L.; Huo, D.Q.; Hou, C.J. Dual-signal aptamer sensor based on polydopamine-gold nanoparticles and exonuclease I for ultrasensitive malathion detection. Sens. Actuators B Chem. 2019, 287, 428–436. [Google Scholar] [CrossRef]
- Tianfei, D. Application of Enzyme Technology in Food Processing and Testing. IOP Conf. Ser. Earth Environ. Sci. 2020, 546, 052066. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, L.; Tang, L.; Peng, B.; Huang, H.W.; Wang, J.J.; Yu, J.F.; Ouyang, X.L.; Tan, J.S. Ultrathin PtNi nanozyme based self-powered photoelectrochemical aptasensor for ultrasensitive chloramphenicol detection. Biosens. Bioelectron. 2019, 146, 111756. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.W.; Shi, X.H. Recent advances in nanozyme research. Adv. Mater. 2018, 31, 1805368. [Google Scholar] [CrossRef]
- Jiang, D.W.; Ni, D.L.; Rosenkrans, Z.T.; Huang, P.; Yan, X.Y.; Cai, W.B. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Wu, J.J.X.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artifificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.W.; Qu, X.G. New insights into nanomaterials combating bacteria: ROS and beyond. Sci. China Life Sci. 2019, 62, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.J.; Zhang, M.; Lou, Z.P.; Zhou, M.; Wang, P.; Wei, H. Engineering nanoceria for enhanced peroxidase mimics: A solid solution strategy. ChemCatChem 2019, 11, 737–743. [Google Scholar] [CrossRef]
- Qi, G.H.; Wang, Y.; Zhang, B.Y.; Sun, D.; Fu, C.C.; Xu, W.Q.; Xu, S.P. Glucose oxidase probe as a surface-enhanced Raman scattering sensor for glucose. Anal. Bioanal. Chem. 2016, 408, 7513–7520. [Google Scholar] [CrossRef]
- Wu, J.H.; Yang, Q.T.; Li, Q.; Li, H.Y.; Li, F. Two-Dimensional MnO2 Nanozyme-Mediated Homogeneous Electrochemical Detection of Organophosphate Pesticides without the Interference of H2O2 and Color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef]
- Hu, W.C.; Pang, J.; Biswas, S.; Wang, K.; Wang, C.; Xia, X.H. Ultrasensitive Detection of Bacteria Using a 2D MOF Nanozyme-Amplified Electrochemical Detector. Anal. Chem. 2021, 93, 8544–8552. [Google Scholar] [CrossRef]
- Arvand, M.; Kermanian, M.; Zanjanchi, M.A. Direct determination of aluminium in foods and pharmaceutical preparations by potentiometry using an AlMCM-41 modified polymeric membrane sensor. Electrochim. Acta 2010, 55, 6946–6952. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.Z.; Hou, C.J.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef]
- Rapini, R.; Cincinelli, A.; Marrazza, G. Acetamiprid multidetection by disposable electrochemical DNA aptasensor. Talanta 2016, 161, 15–21. [Google Scholar] [CrossRef]
- Jiao, Y.C.; Jia, H.Y.; Guo, Y.M.; Zhang, H.Y.; Wang, Z.Q.; Sun, X.; Zhao, J. An ultrasensitive aptasensor for chlorpyrifos based on ordered mesoporous carbon/ferrocene hybrid multiwalled carbon nanotubes. RSC Adv. 2016, 6, 58541–58548. [Google Scholar] [CrossRef]
- Liu, Q.; Huan, J.; Dong, X.Y.; Qian, J.; Hao, N.; You, T.Y.; Mao, H.P.; Wang, K. Resonance energy transfer from CdTe quantum dots to gold nanorods using MWCNTs/rGO nanoribons as efficient signal amplifier for fabricating visible-light-driven “on-off-on” photoelectrochemical acetamiprid aptasensor. Sens. Actuators B Chem. 2016, 235, 647–654. [Google Scholar] [CrossRef]
- Qiao, Y.F.; Li, J.; Li, H.B.; Fang, H.L.; Fan, D.H.; Wang, W. A label-free photoelectrochemical aptasensor for bisphenol a based on surface plasmon resonance of gold nanoparticle-sensitized ZnO nanopencils. Biosens. Bioelectron. 2016, 86, 315–320. [Google Scholar] [CrossRef]
- Chen, D.; Yang, M.; Zheng, N.J.; Xie, N.; Liu, D.L.; Xie, C.F.; Yao, D.S. A novel aptasensor for electrochemical detection of ractopamine, clenbuterol, salbutamol, phenylethanolamine and procaterol. Biosens. Bioelectron. 2016, 80, 525–531. [Google Scholar] [CrossRef]
- Li, H.B.; Qiao, Y.F.; Li, J.; Fang, H.L.; Fan, D.H.; Wang, W. A sensitive and label-free photoelectrochemical aptasensor using Co-doped ZnO diluted magnetic semiconductor nanoparticles. Biosens. Bioelectron. 2016, 77, 378–384. [Google Scholar] [CrossRef]
- Prabhakar, N.; Thakur, H.; Bharti, A.; Kaur, N. Chitosan-iron oxide nanocomposite based electrochemical aptasensor for determination of malathion. Anal. Chim. Acta. 2016, 939, 108–116. [Google Scholar] [CrossRef]
- Song, H.Y.; Kang, T.F.; Li, N.N.; Lu, L.P.; Cheng, S.Y. Highly sensitive voltammetric determination of kanamycin based on aptamer sensor for signal amplification. Anal. Methods 2016, 8, 3366–3372. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.H.; Xu, Q.; Cao, Y.T.; Chen, Y.J. An electrochemical aptasensor for multiplex antibiotics detection based on metal ions doped nanoscale MOFs as signal tracers and RecJF exonuclease-assisted targets recycling amplification. Talanta 2016, 161, 867–874. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhang, H.R.; Yan, Z.D.; Li, T.H.; Chen, Y.J.; Xu, Q.; Jiang, Q.L. Electrochemical simultaneous assay of chloramphenicol and PCB72 using magnetic and aptamer-modified quantum dot-encoded dendritic nanotracers for signal amplification. Microchim. Acta 2016, 183, 1099–1106. [Google Scholar] [CrossRef]
- Wang, H.Z.; Wang, Y.; Liu, S.; Yu, J.H.; Guo, Y.; Xu, Y.; Huang, J.D. Signal-on electrochemical detection of antibiotics at zeptomole level based on target-aptamer binding triggered multiple recycling amplification. Biosens. Bioelectron. 2016, 80, 471–476. [Google Scholar] [CrossRef]
- Yan, Z.D.; Gan, N.; Li, T.H.; Cao, Y.T.; Chen, Y.J. A sensitive electrochemical aptasensor for multiplex antibiotics detection based on high-capacity magnetic hollow porous nanotracers coupling exonuclease-assisted cascade target recycling. Biosens. Bioelectron. 2016, 78, 51–57. [Google Scholar] [CrossRef]
- Ge, L.; Li, H.N.; Du, X.J.; Zhu, M.Y.; Chen, W.; Shi, T.Y.; Hao, N.; Liu, Q.; Wang, K. Facile one-pot synthesis of visible light-responsive BiPO4/nitrogen doped graphene hydrogel for fabricating label-free photoelectrochemical tetracycline aptasensor. Biosens. Bioelectron. 2018, 111, 131–137. [Google Scholar] [CrossRef]
- Danesh, N.M.; Ramezani, M.; Emrani, A.S.; Abnous, K.; Taghdisi, S.M. A novel electrochemical aptasensor based on arch-shape structure of aptamer complimentary strand conjugate and exonuclease I for sensitive detection of streptomycin. Biosens. Bioelectron. 2016, 75, 123–128. [Google Scholar] [CrossRef]
- Yin, Y.; Qin, X.L.; Wang, Q.C.; Yin, Y. A novel electrochemical aptasensor for sensitive detection of streptomycin based on gold nanoparticle functionalized magnetic multi-walled carbon nanotubes and nanoporous PtTi alloy. RSC Adv. 2016, 6, 39401–39408. [Google Scholar] [CrossRef]
- Wang, X.Z.; Dong, S.S.; Gai, P.P.; Duan, R.; Li, F. Highly sensitive homogeneous electrochemical aptasensor for antibiotic residues detection based on dual recycling amplification strategy. Biosens. Bioelectron. 2016, 15, 49–54. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, W.J.; Pei, M.S.; Ding, F. GR-Fe3O4NPs and PEDOT-AuNPs composite based electrochemical aptasensor for the sensitive detection of penicillin. Anal. Methods 2016, 8, 4391–4397. [Google Scholar] [CrossRef]
- Li, K.X.; Qiao, X.J.; Zhao, H.Y.; He, Y.P.; Sheng, Q.L.; Yue, T.L. Ultrasensitive and label-free electrochemical aptasensor based on carbon dots-black phosphorus nanohybrid for the detection of Ochratoxins A. Microchem. J. 2021, 168, 106378. [Google Scholar] [CrossRef]
- Hao, N.; Jiang, L.; Qian, J.; Wang, K. Ultrasensitive electrochemical ochratoxin a aptasensor based on CdTe quantum dots functionalized graphene/Au nanocomposites and magnetic separation. J. Electroanal. Chem. 2016, 781, 332–338. [Google Scholar] [CrossRef]
- Zheng, W.L.; Teng, J.; Cheng, L.; Ye, Y.W.; Pan, D.D.; Wu, J.J.; Xue, F.; Liu, G.D.; Chen, W. Hetero-enzyme-based two-round signal amplification strategy for trace detection of aflatoxin B1 using an electrochemical aptasensor. Biosens. Bioelectron. 2016, 80, 574–581. [Google Scholar] [CrossRef]
- Goud, K.Y.; Catanante, G.; Hayat, A.; Satyanarayana, M.; Gobi, K.V.; Marty, J.L. Disposable and portable electrochemical aptasensor for label free detection of aflatoxin B1 in alcoholic beverages. Sens. Actuators B Chem. 2016, 235, 466–473. [Google Scholar] [CrossRef]
- Istamboulié, G.; Paniel, N.; Zara, L.; Granados, L.R.; Barthelmebs, L.; Noguer, T. Development of an impedimetric aptasensor for the determination of aflatoxin M1 in milk. Talanta 2016, 146, 464–469. [Google Scholar] [CrossRef]
- Mishra, R.K.; Hayat, A.; Catanante, G.; Istamboulie, G.; Marty, J.L. Sensitive quantitation of ochratoxin a in cocoa beans using differential pulse voltammetry based aptasensor. Food Chem. 2016, 192, 799–804. [Google Scholar] [CrossRef]
- Bagheryan, Z.; Raoof, J.B.; Golabi, M.; Turner, A.P.F.; Beni, V. Diazonium-based impedimetric aptasensor for the rapid label-free detection of Salmonella typhimurium in food sample. Biosens. Bioelectron. 2016, 80, 566–573. [Google Scholar] [CrossRef] [Green Version]
- Jia, F.; Duan, N.; Wu, S.J.; Dai, R.T.; Wang, Z.P.; Li, X.M. Impedimetric Salmonella aptasensor using a glass carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim. Acta 2016, 183, 337–344. [Google Scholar] [CrossRef]
- Sheikhzadeh, E.; Chamsaz, M.; Turner, A.P.F.; Jager, E.W.H.; Beni, V. Label-free impedimetric biosensor for Salmonella typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens. Bioelectron. 2016, 80, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Solanas, A.; Patsakis, C.; Conti, M.; Vlachos, I.; Ramos, V.; Falcone, F.; Postolache, O.; Perez-Martinez, P.; Pietro, R.D.; Perrea, D.; et al. Smart health: A context-aware health paradigm within smart cities. IEEE Commun. Mag. 2014, 52, 74–81. [Google Scholar] [CrossRef]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-based food diagnostic technologies: A review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Tsagkaris, A.S.; Dillon, M.J.; Hajslova, J.; Elliott, C.T. Smartphone-based optical assays in the food safety field. Trends Analyt Chem. 2020, 129, 115934. [Google Scholar] [CrossRef]
- Ross, G.M.S.; Bremer, M.G.E.G.; Nielen, M. Consumer-friendly food allergen detection: Moving towards smartphone-based immunoassays. Anal. Bioanal. Chem. 2018, 410, 5353–5371. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.Y.; Cui, Y.; Cai, Z.; Li, L.L. Applications of smartphone-based colorimetric biosensors. Biosens. Bioelectron. X 2022, 11, 100173. [Google Scholar] [CrossRef]
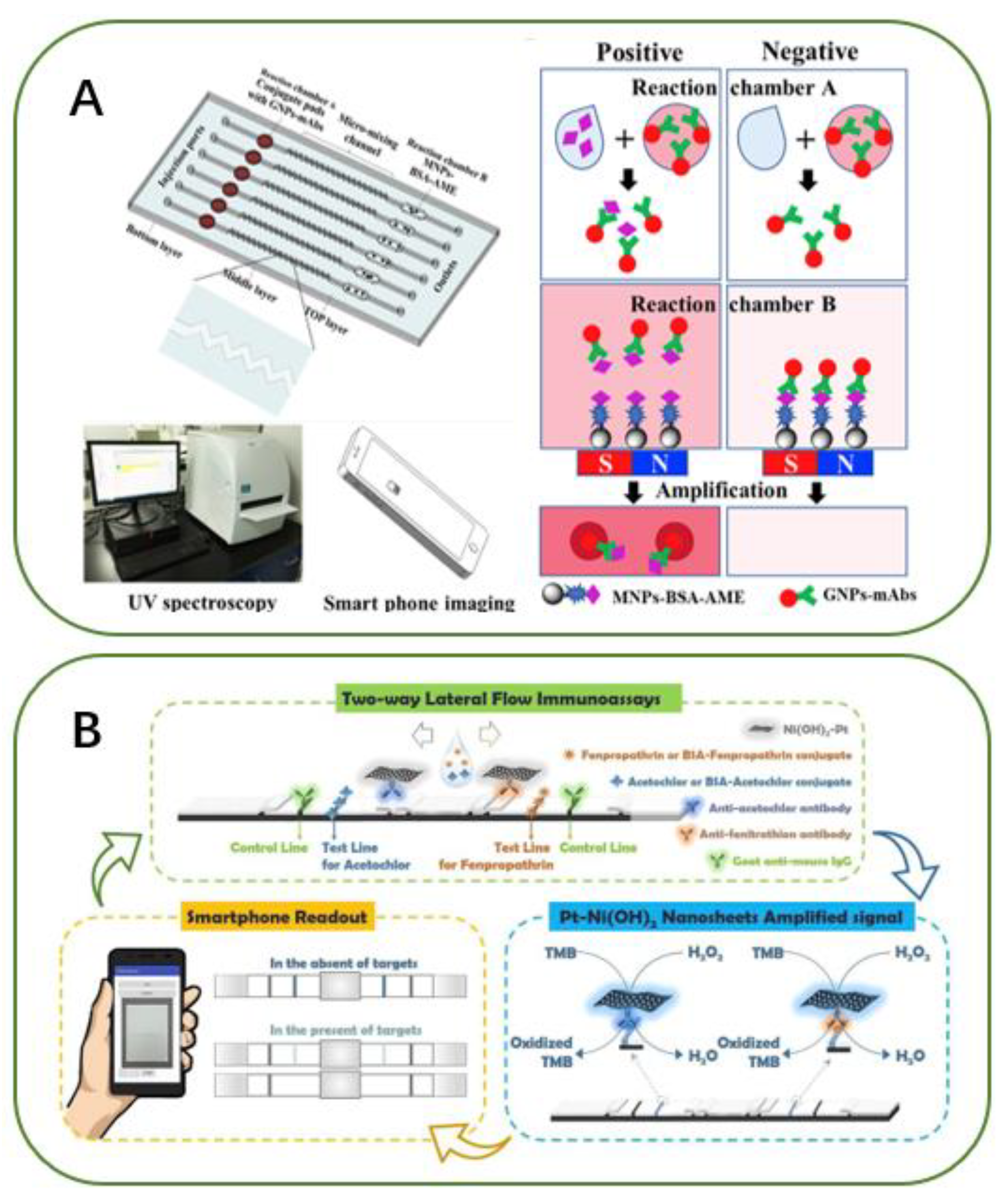
- Man, Y.; Li, A.; Li, B.R.; Liu, J.; Pan, L.G. A microfluidic colorimetric immunoassay for sensitive detection of altenariol monomethyl ether by UV spectroscopy and smart phone imaging. Anal. Chim. Acta 2019, 1092, 75–84. [Google Scholar] [CrossRef]
- Cheng, N.; Shi, Q.R.; Zhu, C.Z.; Li, S.Q.; Lin, Y.H.; Du, D. Pt–Ni(OH)2 nanosheets amplified two-way lateral flow immunoassays with smartphone readout for quantification of pesticides. Biosens. Bioelectron. 2019, 142, 111498. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, J.; Bonadonna, P. Pocket mobile smartphone system for the point-of-care submandibular ultrasonography. Am. J. Emerg. Med. 2013, 31, 573–577. [Google Scholar] [CrossRef]
- Xu, X.Y.; Akay, A.; Wei, H.L.; Wang, S.Q.; Pingguan-Murphy, B.; Erlandsson, B.E.; Li, X.J.; Lee, W.G.; Hu, J.; Wang, L.; et al. Advances in Smartphone-Based Point-of-Care Diagnostics. Proc. IEEE 2015, 103, 236–247. [Google Scholar] [CrossRef]
- Sajed, S.; Arefi, F.; Kolahdouz, M.; Sadeghi, M.A. Improving sensitivity of mercury detection using learning based smartphone colorimetry. Sens. Actuators B Chem. 2019, 298, 126942. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Z.Q.; Zhang, Y.J.; Ma, X.H.; Iqbal, M.Z.; Miao, L.J.; Zhou, Z.W.; Shen, Z.Y.; Wu, A.G. High-Performance Colorimetric Detection of Thiosulfate by Using Silver Nanoparticles for Smartphone-Based Analysis. ACS Sens. 2017, 2, 1152–1159. [Google Scholar] [CrossRef]
- Zhang, J.L.; Khan, I.; Zhang, Q.W.; Liu, X.H.; Dostalek, J.; Liedberg, B.; Wang, Y. Lipopolysaccharides detection on a grating-coupled surface plasmon resonance smartphone biosensor. Biosens. Bioelectron. 2018, 99, 312–317. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, B.; Huang, H.C.; Jian, D.; Wu, X.P.; Xue, L.; Wang, S.Y.; Liu, F. On-site quantitative Hg2+ measurements based on selective and sensitive fluorescence biosensor and miniaturized smartphone fluorescence microscope. Biosens. Bioelectron. 2019, 132, 238–247. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.L.; Zhuo, Y.X.; Song, D.; Li, C.S.; Zhu, A.N.; Long, F. Reusable smartphone-facilitated mobile fluorescence biosensor for rapid and sensitive on-site quantitative detection of trace pollutants. Biosens. Bioelectron. 2022, 199, 113863. [Google Scholar] [CrossRef]
- Zeinhom, M.M.A.; Wang, Y.J.; Sheng, L.N.; Du, D.; Li, L.; Zhu, M.J.; Lin, Y.H. Smart phone based immunosensor coupled with nanoflower signal amplification for rapid detection of Salmonella Enteritidis in milk, cheese and water. Sens. Actuators B Chem. 2018, 261, 75–82. [Google Scholar] [CrossRef]
- Zeinhom, M.M.A.; Wang, Y.J.; Song, Y.; Zhu, M.J.; Lin, Y.H.; Du, D. A portable smart-phone device for rapid and sensitive detection of E. coli O157:H7 in Yoghurt and Egg. Biosens. Bioelectron. 2018, 99, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, S.W.; Yu, T.; Dai, Z.M.; Wei, Q.S. An Aptamer-Based Fluorescent Sensor Array for Rapid Detection of Cyanotoxins on a Smartphone. Anal. Chem. 2019, 91, 10448–10457. [Google Scholar] [CrossRef]
- Wu, F.Y.; Wang, M. A Portable Smartphone-Based Sensing System Using a 3D-Printed Chip for On-Site Biochemical Assays. Sensors 2018, 18, 4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, N.; Song, Y.; Zeinhom, M.M.A.; Chang, Y.C.; Sheng, L.; Li, H.L.; Du, D.; Li, L.; Zhu, M.J.; Luo, Y.B.; et al. Nanozyme-mediated dual immunoassay integrated with smartphone for use in simultaneous detection of pathogens. ACS Appl. Mater. Interfaces 2017, 9, 40671–40680. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Song, Y.; Fu, Q.Q.; Du, D.; Luo, Y.B.; Wang, Y.; Xu, W.T.; Lin, Y.H. Aptasensor based on fluorophore-quencher nano-pair and smartphone spectrum reader for on-site quantification of multi-pesticides. Biosens. Bioelectron. 2018, 117, 75–83. [Google Scholar] [CrossRef] [PubMed]
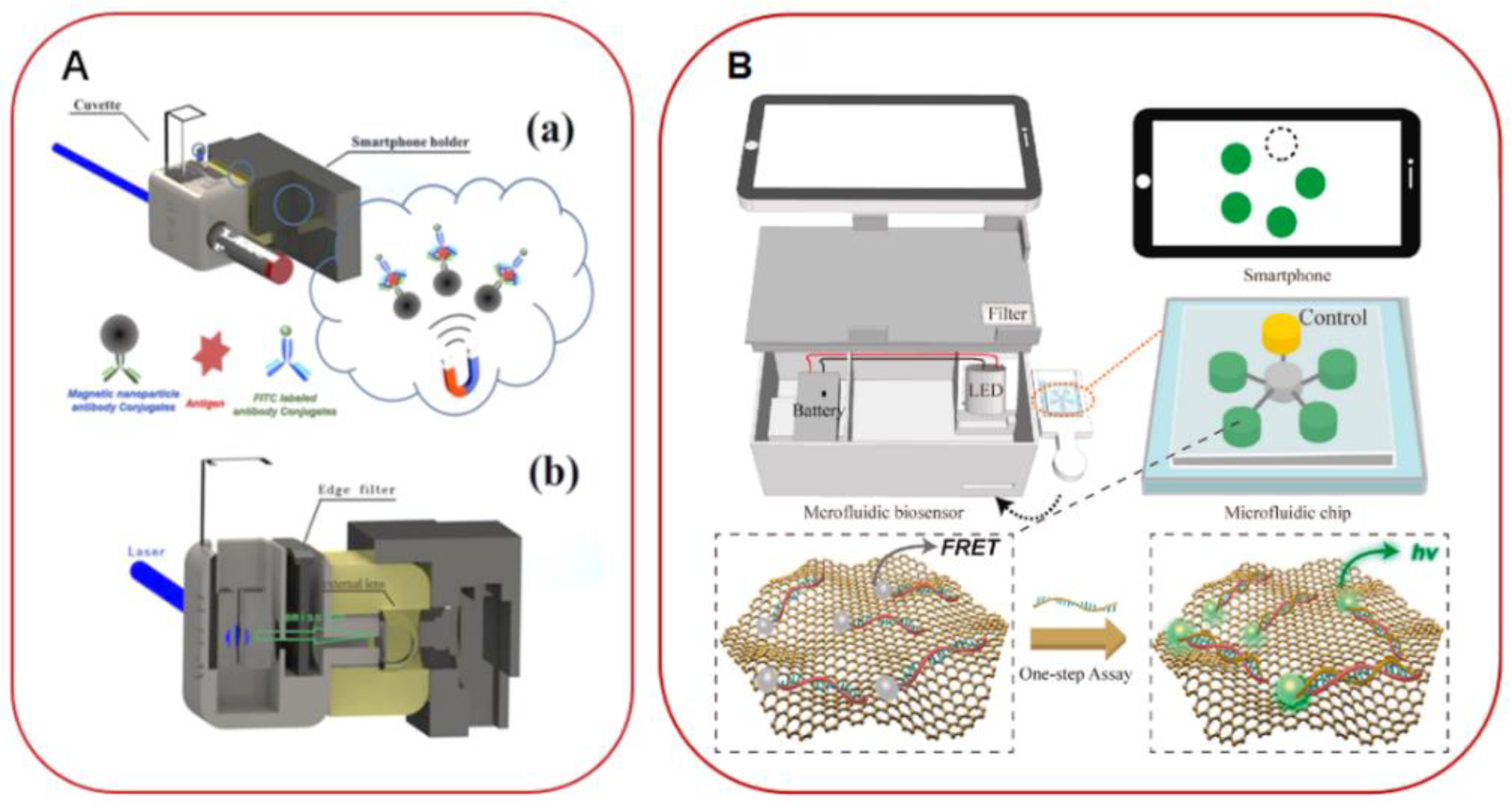
- Xing, G.W.; Li, N.; Lin, H.F.; Shang, Y.T.; Pu, Q.S.; Lin, J.M. Microfluidic biosensor for one-step detection of multiplex foodborne bacteria ssDNA simultaneously by smartphone. Talanta 2022, 253, 123980. [Google Scholar] [CrossRef]
- Zhang, D.M.; Jiang, J.; Chen, J.Y.; Zhang, Q.; Lu, Y.L.; Yao, Y.; Li, S.; Liu, G.L.; Liu, Q.J. Smartphone-based portable biosensing system using impedance measurement with printed electrodes for 2, 4, 6-trinitrotoluene (TNT) detection. Biosens. Bioelectron. 2015, 70, 81–88. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Rajaji, U.; Chen, S.M.; Liu, T.Y.; Filho, J.I.D.O.; Chang, Y.S. Fabrication of thulium metal–organic frameworks based smartphone sensor towards arsenical feed additive drug detection: Applicable in food safety analysis. Electrochim. Acta 2021, 401, 139487. [Google Scholar] [CrossRef]
- Fan, L.; Huang, J.J.; Liao, J.J. Competitive smartphone-based portable electrochemical aptasensor system based on an MXene/cDNA-MB probe for the determination of Microcystin-LR. Sens. Actuators B Chem. 2022, 369, 132164. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, C.; Yuan, W.; Liu, Z.Y.; Zhu, L.H.; Li, X.T.; Lu, Y.L.; Chen, Z.T.; Liu, J.L.; Cui, Z.; et al. Smartphone-based battery-free and flexible electrochemical patch for calcium and chloride ions detections in biofluids. Sens. Actuators B Chem. 2019, 297, 126743. [Google Scholar] [CrossRef]
- Ji, D.Z.; Liu, L.; Li, S.; Chen, C.; Lu, Y.L.; Wu, J.J.; Liu, Q. Smartphone-based cyclic voltammetry system with graphene modified screen printed electrodes for glucose detection. Biosens. Bioelectron. 2017, 98, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Hubble, L.J.; Martín, A.; Kumar, R.; Barfidokht, A.; Kim, J.; Musameh, M.M.; Kyratzis, I.L.; Wang, J. Wearable Flexible and Stretchable Glove Biosensor for On-Site Detection of Organophosphorus Chemical Threats. ACS Sens. 2017, 2, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Cheng, C.; Liu, Z.Y.; Yuan, W.; Wu, X.Z.; Lu, Y.L.; Low, S.S.; Liu, J.L.; Zhu, L.H.; Ji, D.Z.; et al. Battery-Free and Wireless Epidermal Electrochemical System with All-Printed Stretchable Electrode Array for Multiplexed In Situ Sweat Analysis. Adv. Mater. Technol. 2019, 4, 1800658. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Q.; Lu, Y.L.; Liu, L.; Ji, D.Z.; Li, S.; Liu, Q.J. Passive and wireless near field communication tag sensors for biochemical sensing with smartphone. Sens. Actuators B Chem. 2017, 246, 748–755. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, P.; Cheng, W.; Yan, K.; Pan, L.J.; Shi, Y.; Yu, G.H. Highly sensitive, printable nanostructured conductive polymer wireless sensor for food spoilage detection. Nano Lett. 2018, 18, 4570–4575. [Google Scholar] [CrossRef]










| Carbon Precursor | Methods | Target | Limit of Detection | |
|---|---|---|---|---|
| Wang et al. [74] | Papaya flesh | Thermal/200 °C/5 h | Escherichia coil | 9.5 × 104 cfu mL−1 |
| Das et al. [75] | Gram shells | Pyrolysis/315 °C/3 h | Escherichia coil | 107 cfu mL−1 |
| Hu et al. [76] | Orange peel | Microwave/900 W/1 min | Escherichia coil | 487 cfu mL−1 |
| Liu et al. [77] | Tomato puree | Microwave/10 min | Vanillin | 24.9 mg kg−1 |
| Bao et al. [78] | Eleocharis dulcis | Hydrothermal/120 °C/5 h | Fe3+ | 560 nM |
| Bandi et al. [79] | Onion waste | Hydrothermal/120 °C/2 h | Fe3+ | 310 nM |
| Shen et al. [80] | Sweet potatoes | Hydrothermal/180 °C/18 h | Fe3+ | 320 nM |
| Zhao et al. [81] | Corn bract | Reflux/100 °C/24 h | Hg2+ | 9.0 nM |
| Bano et al. [82] | Tamarindus indica leaves | Hydrothermal/210 °C/5 h | Hg2+ | 6 nM |
| Tyagi et al. [83] | Lemon peels | Hydrothermal/200 °C/12 h | Cr6+ | 73 nM |
| Kumar et al. [84] | Tulsi leaves | Hydrothermal/180 °C/4 h | Pb2+ | 0.59 nM |
| Bhatt et al. [85] | Tulsi leaves | Hydrothermal/200 °C/4 h | Cr6+ | 4.5 ppb |
| Wen et al. [86] | Pigskin | Hydrothermal/250 °C/2 h | Co2+ | 680 nM |
| Liu et al. [87] | Chocolate | Hydrothermal/200 °C/8 h | Pb2+ | 12.7 nM |
| Analyte | Modified Electrode | Food Samples | Detection Techniques | LOD | |
|---|---|---|---|---|---|
| Rapini et al. [150] | Acetamiprid | PANI/AuNPs/GPSEs | Fruit juice | DPV | 86 nM |
| Jiao et al. [151] | Chlorpyrifos | Fc@MWCNTs/OMC/GCE | Fresh pakchoi, lettuce and leek | CV | 0.33 ng mL−1 |
| Liu et al. [152] | Acetamiprid | CdTe-MWCNTs/ rGONRs/ITO | Apples and tomatoes | PEC | 0.2 pM |
| Qiao et al. [153] | BPA | Au/ZnO/ITO | Milk and drinking water | PEC | 0.5 nM |
| Chen et al. [154] | β-agonists: RAC, CLB, PHL, SAL and PRO | AP-Ago/AuE | Pork sample | DPV | 40 (RAC), 0.35 (CLB), 1.0 (PHL), 0.53 (SAL) and 1.73 (PRO) pg mL−1 |
| Li et al. [155] | Acetamiprid | Co-doped ZnO/ITO | Cucumber | PEC | 0.18 nM |
| Prabhakar et al. [156] | Malathion | CHIT-IO/FTO | Lettuce leaves | DPV | 0.001 ng/mL |
| Song et al. [157] | KMY | HRP-AuNPCdna/Aptamer/AuE | Milk | DPV | 0.005 µg L−1 |
| Chen et al. [158] | KMY and OTC | Dynabead | Milk | SWV | 0.15 and 0.18 pM |
| Chen et al. [159] | CAP | cDNA/GCE | Fish samples | SWV | 0.33 pg mL−1 |
| Wang et al. [160] | KMY | AuE | Milk | DPV | 1.3 f M |
| Yan et al. [161] | CAP and OTC | AuNPs/GCE | Milk | SWV | 0.15 and 0.1 ng mL−1 |
| Ge et al. [162] | TTC | BiPO4/3DNGH/ITO | Milk | PEC | 0.033 nM |
| Danesh et al. [163] | SMY | Apt-CHIT/SPGE | Milk | DPV | 11.4 nM |
| Yin et al. [164] | SMY | NP-PtTi/Au@MWCNTsFe3O4/GCE | Milk | DPV | 7.8 pg mL−1 |
| Wang et al. [165] | AMP | ITO electrode | Milk | DPV | 4.0 pM |
| Zhao et al. [166] | Penicillin | PEDOT-AuNPs/GRFe2O3NPs/GCE | Milk | DPV | 0.057 ng mL−1 |
| Li et al. [167] | OTA | CDs-BP | Wheat and grape juice | EIS | 0.03 fg mL−1 |
| Hao et al. [168] | OTA and FB1 | BFE | Maize | SWV | 5 (OTA); 20 (FB1) pg mL−1 |
| Zheng et al. [169] | AFB1 | TS-AuNPs-cDNA/AuE | Animal feed and food samples | SWV | 0.6 × 10−4 ppt |
| Goud et al. [170] | AFB1 | SPCE | Beer and wine sample | EIS | 0.12 (seqA) and 0.25 (seqB) ng mL−1 |
| Istamboulié et al. [171] | AFM1 | SPCE | Milk | EIS | 1.15 ng L−1 |
| Mishra et al. [172] | OTA | Apt/SPCE | Cocoa beans | DPV | 0.07 ng mL−1 |
| Bagheryan et al. [173] | S. typhimurium | Diazonium-grafting layer modified SPCE | Apple juice sample | EIS | 6 cfu mL−1 |
| Jia et al. [174] | Salmonella | rGO-MWCNT/GCE | Chicken sample | EIS | 25 cfu mL−1 |
| Sheikhzadeh er al. [175] | S. typhimurium | Poly[pyrrole-co-3-carboxylpyrrole]copolymer/AuE | Apple juice sample | EIS | 3 cfu mL−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, X.; He, J.; Yang, R.; Li, Y.; Chen, G.; Xiao, S.; Huang, B.; Yuan, Y.; Sheng, Q.; Yue, T. Recent Advances in Nanomaterial-Based Sensing for Food Safety Analysis. Processes 2022, 10, 2576. https://doi.org/10.3390/pr10122576
Qiao X, He J, Yang R, Li Y, Chen G, Xiao S, Huang B, Yuan Y, Sheng Q, Yue T. Recent Advances in Nanomaterial-Based Sensing for Food Safety Analysis. Processes. 2022; 10(12):2576. https://doi.org/10.3390/pr10122576
Chicago/Turabian StyleQiao, Xiujuan, Jingyi He, Ruixi Yang, Yanhui Li, Gengjia Chen, Sanxiong Xiao, Bo Huang, Yahong Yuan, Qinglin Sheng, and Tianli Yue. 2022. "Recent Advances in Nanomaterial-Based Sensing for Food Safety Analysis" Processes 10, no. 12: 2576. https://doi.org/10.3390/pr10122576





