Evaluation of Carbon Nanotubes Functionalized Polydimethylsiloxane Based Coatings for In-Tube Solid Phase Microextraction Coupled to Capillary Liquid Chromatography
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Equipment
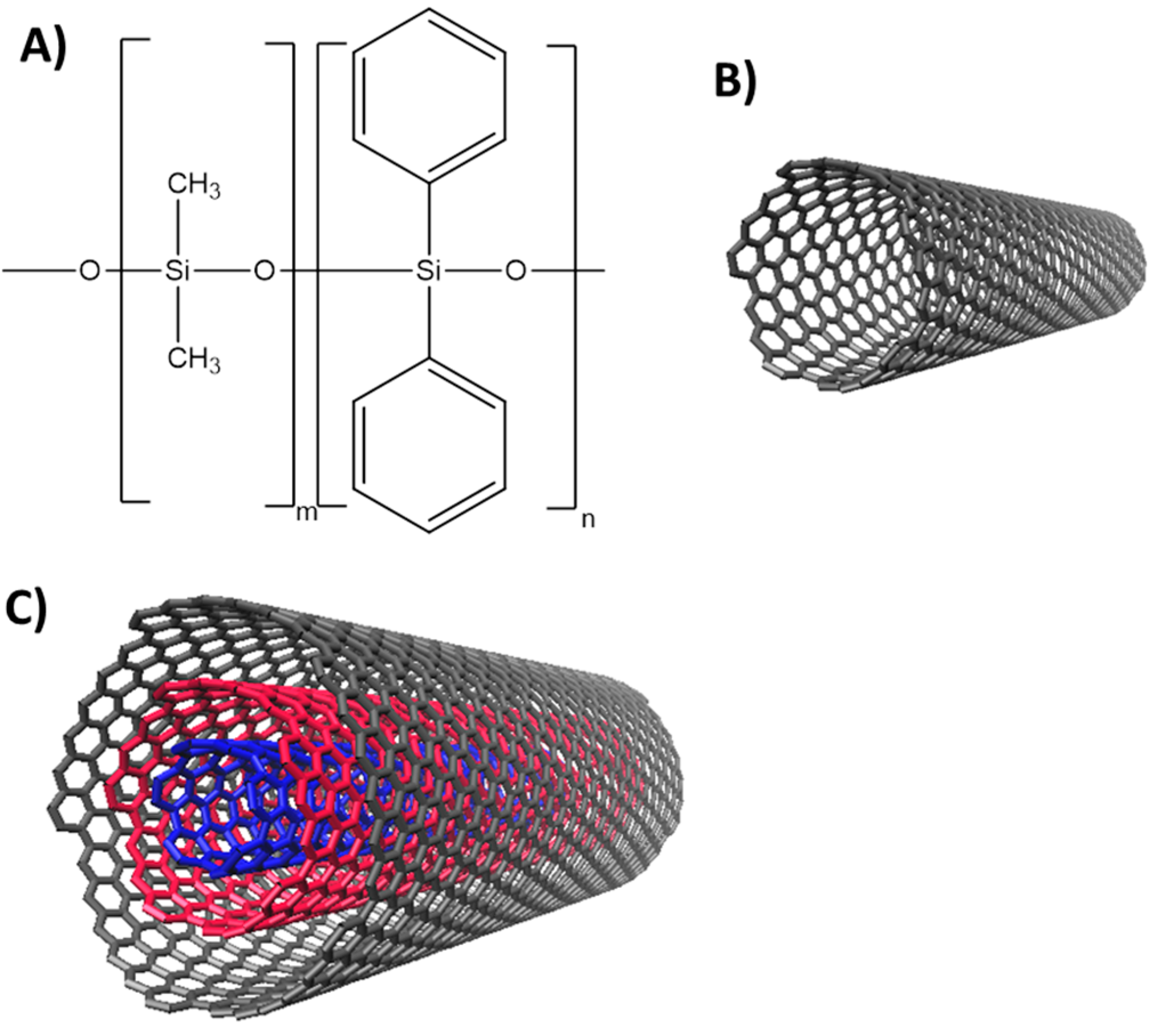
2.3. Functionalization of the Capillary Columns
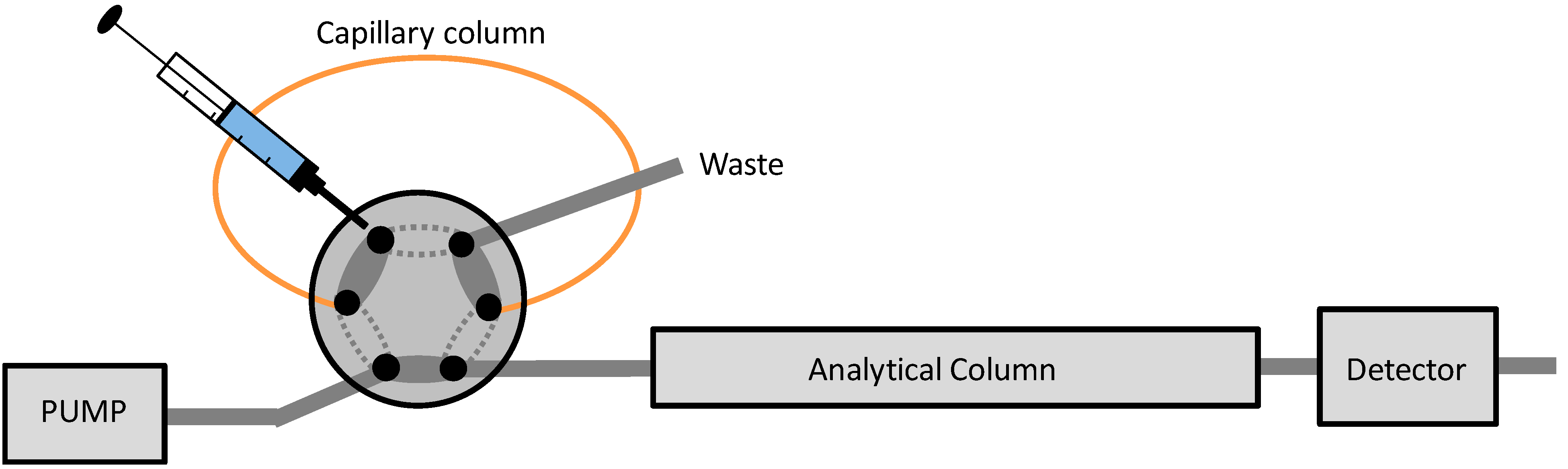
2.4. IT-SPME Procedure and Chromatographic Conditions
3. Results and Discussion
3.1. Characterization of c-CNTs and Capillary Columns

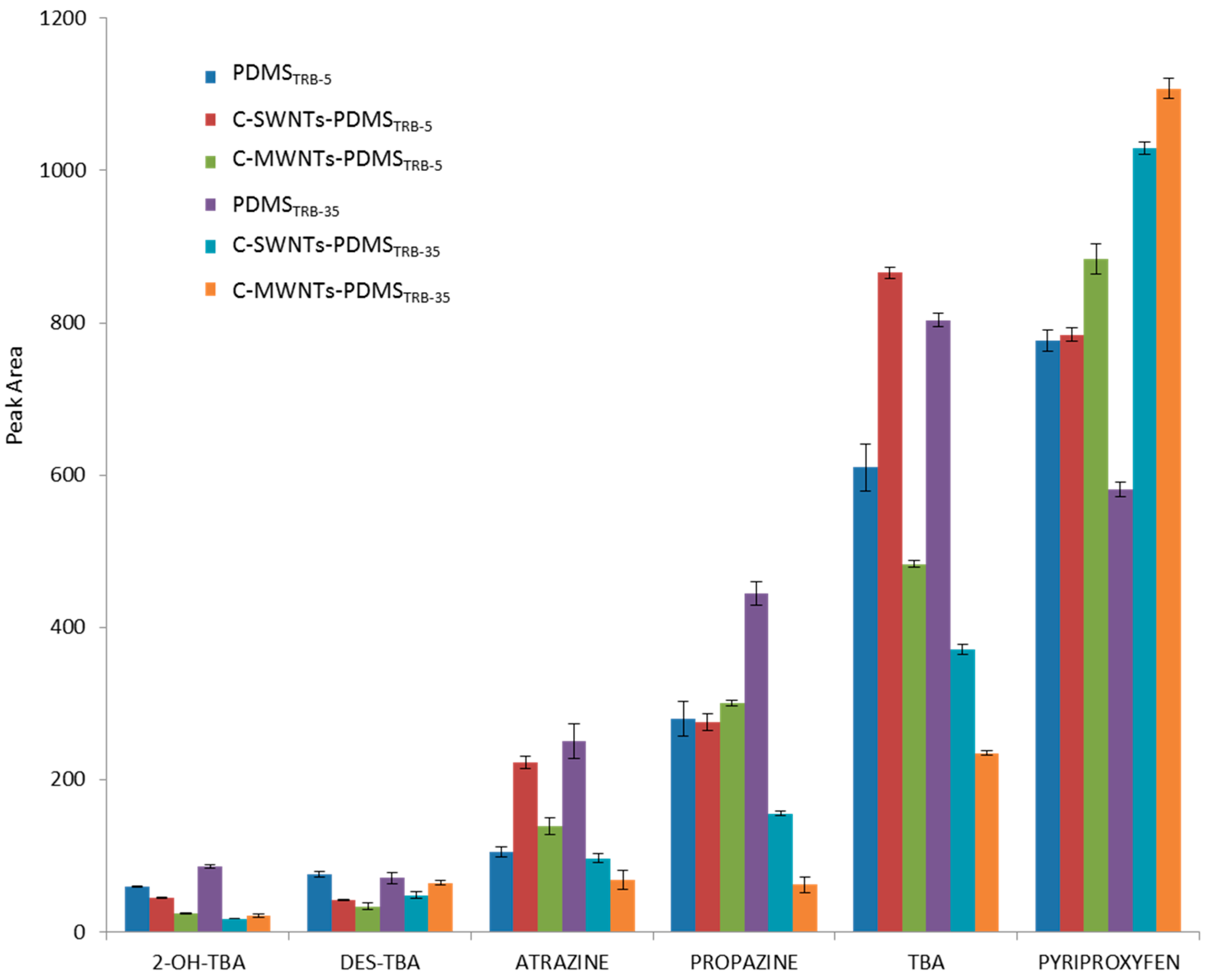
3.2. Study of the Extraction Capability of the c-CNTs-PDMS Coated Capillary Columns for Nitrogen Heterocyclic Compounds
| NAME | STRUCTURE | L.O.D. (µg/L) | |||||
|---|---|---|---|---|---|---|---|
| PDMSTRB-35 | c-SWNTs-PDMSTRB-35 | c-MWNTs-PDMSTRB-35 | PDMSTRB-5 | c-SWNTs-PDMSTRB-5 | c-MWNTs-PDMSTRB-5 | ||
| Terbuthylazine-2-hydroxy (2-OH-TBA) |  | 3.5 | 16 | 14 | 5 | 7 | 12 |
| Terbuthylazine-desethyl (DES-TBA) |  | 4 | 6 | 7 | 4 | 7 | 9 |
| Atrazine |  | 1.2 | 3 | 4 | 3 | 1.3 | 2 |
| Propazine |  | 0.7 | 1.9 | 5 | 1.1 | 1.1 | 2 |
| Terbuthylazine (TBA) |  | 0.4 | 0.8 | 1.3 | 0.5 | 0.4 | 0.6 |
| Pyriproxyfen |  | 0.1 | 0.06 | 0.05 | 0.08 | 0.08 | 0.07 |
| Naphtalene |  | 0.15 | 0.23 | 0.33 | 0.23 | 0.06 | 0.07 |
| Benzo[b]fluoranthene |  | 0.35 | 0.37 | 0.21 | 0.17 | 0.03 | 0.07 |
| Dibenzo[a,h]anthracene |  | 3.85 | 0.33 | 0.83 | 0.88 | 0.14 | 0.29 |
3.3. Study of the Extraction Capability of the c-CNTs-PDMS Capillary Columns for PAHs
3.4. Precision

| RSD (%) | ||||||
|---|---|---|---|---|---|---|
| PDMSTRB 5 | c-SWNTs- | c-MWNTs- | PDMSTRB 35 | c-SWNTs- | c-MWNTs- | |
| PDMSTRB 5 | PDMSTRB 5 | PDMSTRB 35 | PDMSTRB 35 | |||
| 2-OH-TBA | 1.2 | 1.8 | 2 | 4.3 | 1.2 | 6.1 |
| DES-TBA | 1.9 | 1.2 | 6.5 | 5.2 | 4.1 | 3.8 |
| Atrazine | 3.8 | 3.8 | 7.7 | 7.3 | 6 | 6.9 |
| Propazine | 2.3 | 3.9 | 1.1 | 3.4 | 2.2 | 2.1 |
| TBA | 1.5 | 0.9 | 1.1 | 1.6 | 1.8 | 1.2 |
| Pyriproxyfen | 1.8 | 1.2 | 2.2 | 1.6 | 0.8 | 1.2 |
| Naphthalene | 7.3 | 5.7 | 4.9 | 7.6 | 6.5 | 5.1 |
| Benzo[b]fluoranthene | 8.5 | 2.8 | 7.7 | 2.7 | 1.5 | 4 |
| Dibenz[a,h]anthracene | 4.2 | 6.8 | 6.3 | 6.4 | 8.6 | 4.7 |
3.5. Evaluation of the Preconcentration Ration Achieved with the c-CNTs-PDMS Capillary Coatings
| Log Kow [32] | c-SWNTs-PDMSTRB 5 | c-MWNTs- PDMSTRB 5 | c-SWNTs-PDMSTRB 35 | c-MWNTs-PDMSTRB 35 | |
|---|---|---|---|---|---|
| 2-OH-TBA | 1.82 | 0.6 | 0.4 | 0.2 | 0.2 |
| DES-TBA | 2.30 | 0.8 | 0.4 | 0.7 | 0.9 |
| Atrazine | 2.61 | 2.1 | 1.3 | 0.4 | 0.3 |
| Propazine | 2.93 | 1.0 | 1.1 | 0.4 | 0.1 |
| TBA | 3.21 | 1.4 | 0.8 | 0.5 | 0.3 |
| Naphthalene | 3.37 | 4.0 | 3.2 | 0.6 | 0.5 |
| Pyriproxyfen | 5.37 | 1.0 | 1.1 | 1.8 | 1.9 |
| Benzo[b]fluoranthene | 6.04 | 4.9 | 2.4 | 0.9 | 1.7 |
| Dibenz[a,h]anthracene | 6.86 | 6.3 | 3.0 | 1.2 | 4.6 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Risticevic, S.; Niri, V.H.; Vuckovic, D.; Pawliszyn, J. Recent developments in solid phase microextraction. Anal. Bioanal. Chem. 2009, 393, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Chen, Y.-H.; Ma, J.-F.; Hu, M.-J.; Li, Y.; Fang, J.-H.; Gao, H.-Q. A novel ionic-liquid-modifies organic-polymer as the sorbent for in-tube solid-phase microextraction of acidic food additives. Anal. Bioanal. Chem. 2014, 406, 4955–4963. [Google Scholar] [CrossRef] [PubMed]
- Aufartová, J.; Mahugo-Santana, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Novákova, L.; Solich, P. Determination of steroids hormones in biological and environmental samples using Green microextraction techniques. Anal. Chim. Acta 2011, 704, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.E.C.; Melo, L.P. Selective capillary coating materials for in-tube solid-phase microextraction coupled to liquid chromatography to determine drugs and biomarkers in biological samples: A review. Anal. Chim. Acta 2014, 826, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moliner-Martinez, Y.; Herráez-Hernández, R.; Verdú-Andrés, J.; Molins-Legua, C.; Campins-Falcó, P. Recent advances of in-tube solid-phase microextraction. Trends Anal. Chem. 2015. [CrossRef]
- Eisert, R.; Pawliszyn, J. Automated in-tube solid phase microextraction coupled to high-performance liquid chromatography. Anal. Chem. 1997, 69, 3140–3147. [Google Scholar] [CrossRef]
- Kataoka, H.; Ishizaki, A.; Nonaka, Y.; Saito, K. Developments and applications of capillary microextraction techniques: A review. Anal. Chim. Acta 2009, 655, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Ji, Y.S.; Zhang, H.X.; Liu, M.C. Highly sensitive analysis of substituted aniline compounds in water samples by using oxidized multiwalled carbon nanotubes as an in-tube solid phase microextraction medium. J. Chromatogr. A 2008, 1212, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fuenzalida, R.A.; Moliner-Martinez, Y.; Verdú-Andrés, J.; Molins-Legua, C.; Herráez-Hernández, R.; Campins-Falcó, P. Nanoparticle Microextraction in Sample Extraction Techniques for Biological Samples: Recent Advances and Novel Applications; De Vooght-Johnson, R., Ed.; Future Science Ltd, unitec House, 2 Albert Place: London, UK. (in press)
- Moliner-Martínez, Y.; Prima-Garcia, H.; Ribera, A.; Coronado, E.; Campíns-Falcó, P. Magnetic in-tube solid phase microextraction. Anal. Chem. 2012, 84, 7233–7240. [Google Scholar] [CrossRef] [PubMed]
- Campíns-Falco, P.; Coronado-Miralles, E.; Moliner-Martínez, Y.; Ribera, A.; Prima-García, H. Magnetic in-tube solid phase microextraction. Patent 201100823 Spain, 2014. International application PCT/ES2012/000205. [Google Scholar]
- Lijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, J.; Tian, J.; Zhu, F.; Zeng, F.; Su, C.; Ouyang, G. New materials in solid-phase microextraction. TrAC-Trend. Anal. Chem. 2013, 47, 68–83. [Google Scholar] [CrossRef]
- Valcarcel, M.; Cardenas, S.; Simonet, B.M.; Moliner-Martínez, Y.; Lucena, R. Carbon nanoestructures as sorbent materials in analytical processes. TrAC-Trend. Anal. Chem. 2008, 27, 34–43. [Google Scholar] [CrossRef]
- Pyrzynska, K. Carbon nanotubes as sorbent in the analysis of pesticides. Chemosphere 2011, 83, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Kueseng, P.; Pawliszyn, J. Carboxylated multiwalled carbon nanotubes/polydimethylsiloxane, anew coating for 96-blade solid-phase microextraction for determination of phenolic compounds in water. J. Chromatogr. A 2013, 1317, 199–202. [Google Scholar] [CrossRef]
- Song, X.Y.; Ha, W.; Chen, J.; Shi, Y.P. Application of beta-cyclodextrin-modified, carbon nanotube-reinforced hollow fiber to solid-phase microextraction of plant hormones. J. Chromatogr. A 2014, 1374, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, Y.; Jing, R.; Jiang, X.; Tian, M. Detection of Organophosphorous Pesticides in Soil Sample with Multiwalled Carbon Nanotubes Coating SPME Fiber. B. Environ. Contam. Tox. 2014, l93, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Bahzadi, M.; Noroozian, E.; Mirzaei, M. A novel coating based on carbon nanotubes/poly-ortho-phenylenediamine composite for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. Talanta 2013, 108, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, J.; Tian, J.; Zhu, F.; Zeng, F.; Su, C.; Ouyang, G. New materials in solid-phase microextraction. TrAC-Trend. Anal. Chem. 2013, 47, 68–83. [Google Scholar] [CrossRef]
- Ali, M.; Zanjani, A.; Ovasis, M. Advances for sensitive, rapid and selective extraction in different configurations of solid-phase microextraction. TrAC-Trend. Anal. Chem. 2013, 51, 13–22. [Google Scholar] [CrossRef]
- Ferial, G.; Amirhassan, A.; Robian, Y. Methods for coating solid-phase microextraction fibers with carbon nanotubes. TrAC-Trend. Anal. Chem. 2014, 58, 133–143. [Google Scholar]
- Wang, X.; Li, X.; Li, Z.; Zhang, Y.; Bai, Y.; Liu, H. Online coupling of in-tube solid phase microextraction with direct analysis in real time mass spectrometry for rapid determination of triazine herbicides in water using carbon-nanotubes-incorporated polymer monolith. Anal. Chem. 2014, 86, 4739–4747. [Google Scholar] [CrossRef] [PubMed]
- Moliner-Martinez, Y.; Serra-Mora, P.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campins-Falcó, P. Analysis of polar triazines and degradation products in waters by in-tube solid-phase microextraction and capillary chromatography: an environmentally friendly method. Anal. Bioanal. Chem. 2015, 407, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- EU Parliament. Directive 2008/105/EC of the European Parliament and Council on environmental quality standards in the field of water policy. Official J. Eur. Union 2008, L348, 84–97. [Google Scholar]
- Suarez, B.; Moliner-Martínez, Y.; Cardenas, S.; Simonet, B.M.; Valcárcel, M. Monitoring of carboxylic carbon nanotubes in surface water by using multiwalled carbon nanotube-modified filter as preconcentration unit. Environ. Sci. Technol. 2008, 48, 6100–6104. [Google Scholar] [CrossRef]
- Sombra, L.; Moliner-Martínez, Y.; Cardenas, S.; Valcárcel, M. Carboxylic multi-walled carbón nanotubes as immobilized stationary phase in capillary electrochromatography. Electrophoresis 2008, 29, 3850–3857. [Google Scholar] [CrossRef] [PubMed]
- Slentz, B.E.; Panner, N.A.; Lugowaska, E.; Regnier, F. Nanoliter capillary electrochromatography columns based on collocated monolithic support structures molded in poly(dimethylsiloxane). Electrophoresis 2001, 22, 3736–3743. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Tao, S.; Xing, B. Relative importance of multiple mechanisms in sorption of organic compounds by multiwalled carbon nanotubes. Carbon 2010, 48, 3721–3728. [Google Scholar] [CrossRef]
- Moliner-Martínez, Y.; González-Fuenzalida, R.A.; Herráez-Hernández, R.; Campíns-Falcó, P.; Verdú-Andrés, J. Cleaning sorbents used in matrix solid-phase dispersion with sonication: Application to the estimation of polycyclic aromatic hydrocarbons at ng/g levels in marine sediments. J. Chromatogr. A 2012, 1263, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kragulj, M.; Tričković, J.; Dalmacija, B.; Kukovecz, Á.; Kóya, Z.; Molnar, J.; Rončević, S. Molecular interactions between organic compounds and functionally modified multiwalled carbon nanotubes. Chem. Eng. J. 2013, 225, 144–152. [Google Scholar] [CrossRef]
- Schmitt, P.H.; Garrison, A.W.; Freitage, D.; Kettrup, A. Separation of s-triazine herbicides and their metabolites by capillary zone electrophoresis as a function of pH. J. Chromatogr. A 1996, 723, 169–177. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jornet-Martínez, N.; Serra-Mora, P.; Moliner-Martínez, Y.; Herráez-Hernández, R.; Campíns-Falcó, P. Evaluation of Carbon Nanotubes Functionalized Polydimethylsiloxane Based Coatings for In-Tube Solid Phase Microextraction Coupled to Capillary Liquid Chromatography. Chromatography 2015, 2, 515-528. https://doi.org/10.3390/chromatography2030515
Jornet-Martínez N, Serra-Mora P, Moliner-Martínez Y, Herráez-Hernández R, Campíns-Falcó P. Evaluation of Carbon Nanotubes Functionalized Polydimethylsiloxane Based Coatings for In-Tube Solid Phase Microextraction Coupled to Capillary Liquid Chromatography. Chromatography. 2015; 2(3):515-528. https://doi.org/10.3390/chromatography2030515
Chicago/Turabian StyleJornet-Martínez, Neus, Pascual Serra-Mora, Yolanda Moliner-Martínez, Rosa Herráez-Hernández, and Pilar Campíns-Falcó. 2015. "Evaluation of Carbon Nanotubes Functionalized Polydimethylsiloxane Based Coatings for In-Tube Solid Phase Microextraction Coupled to Capillary Liquid Chromatography" Chromatography 2, no. 3: 515-528. https://doi.org/10.3390/chromatography2030515






