Monoliths in Bioprocess Technology
Abstract
:1. Introduction
2. Advantages and Limitations of Monoliths
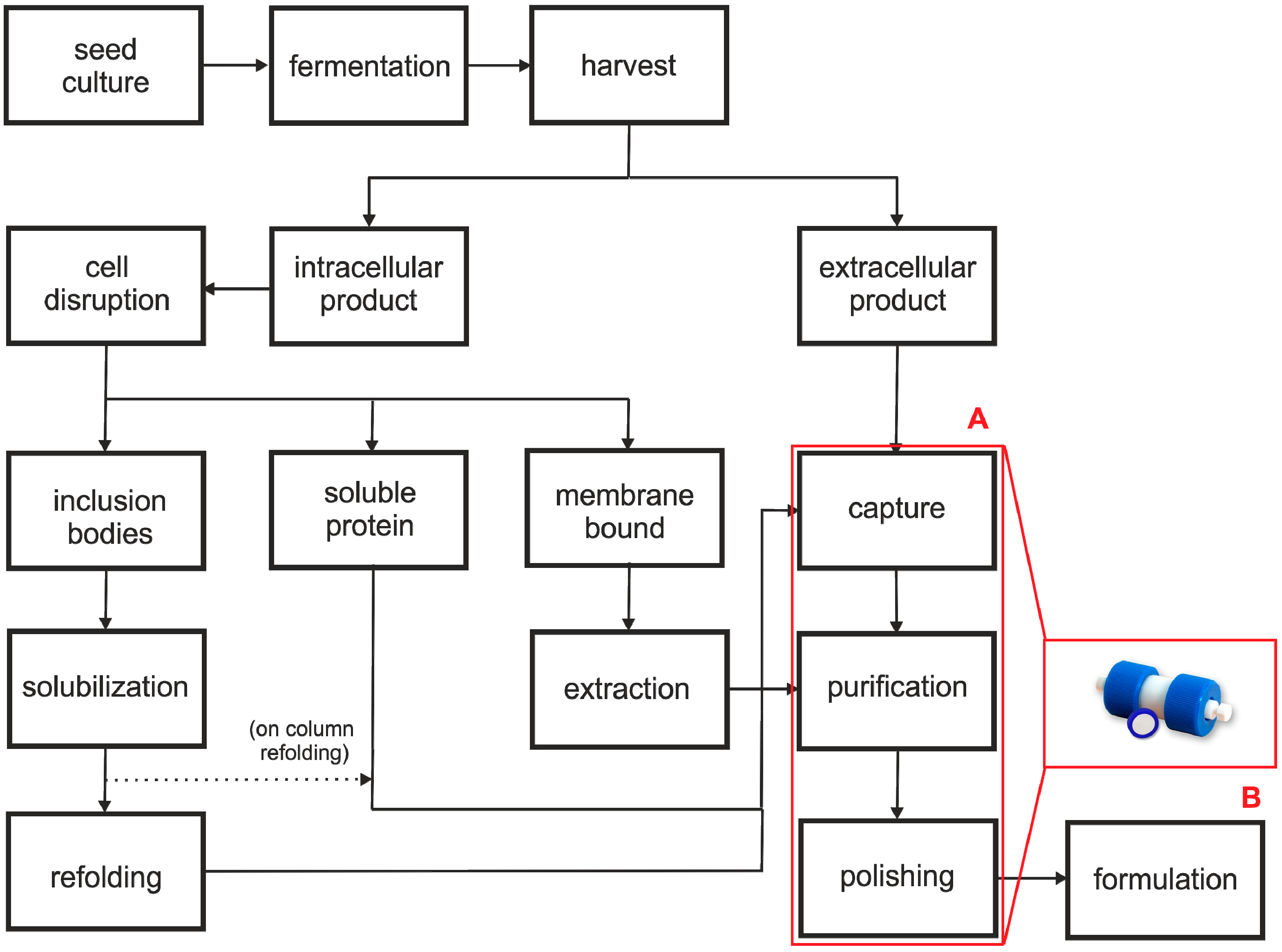
2.1. Advantages of Monolithic Columns
2.1.1. Convective Flow
2.1.2. Porosity
2.1.3. Adsorption Area and Dynamic Binding Capacity
2.1.4. Separation Time
2.1.5. Stationary Phase
2.1.6. Customizability
2.1.7. Scalability
2.2. Limitations of Monolithic Columns
3. Applications of Monoliths
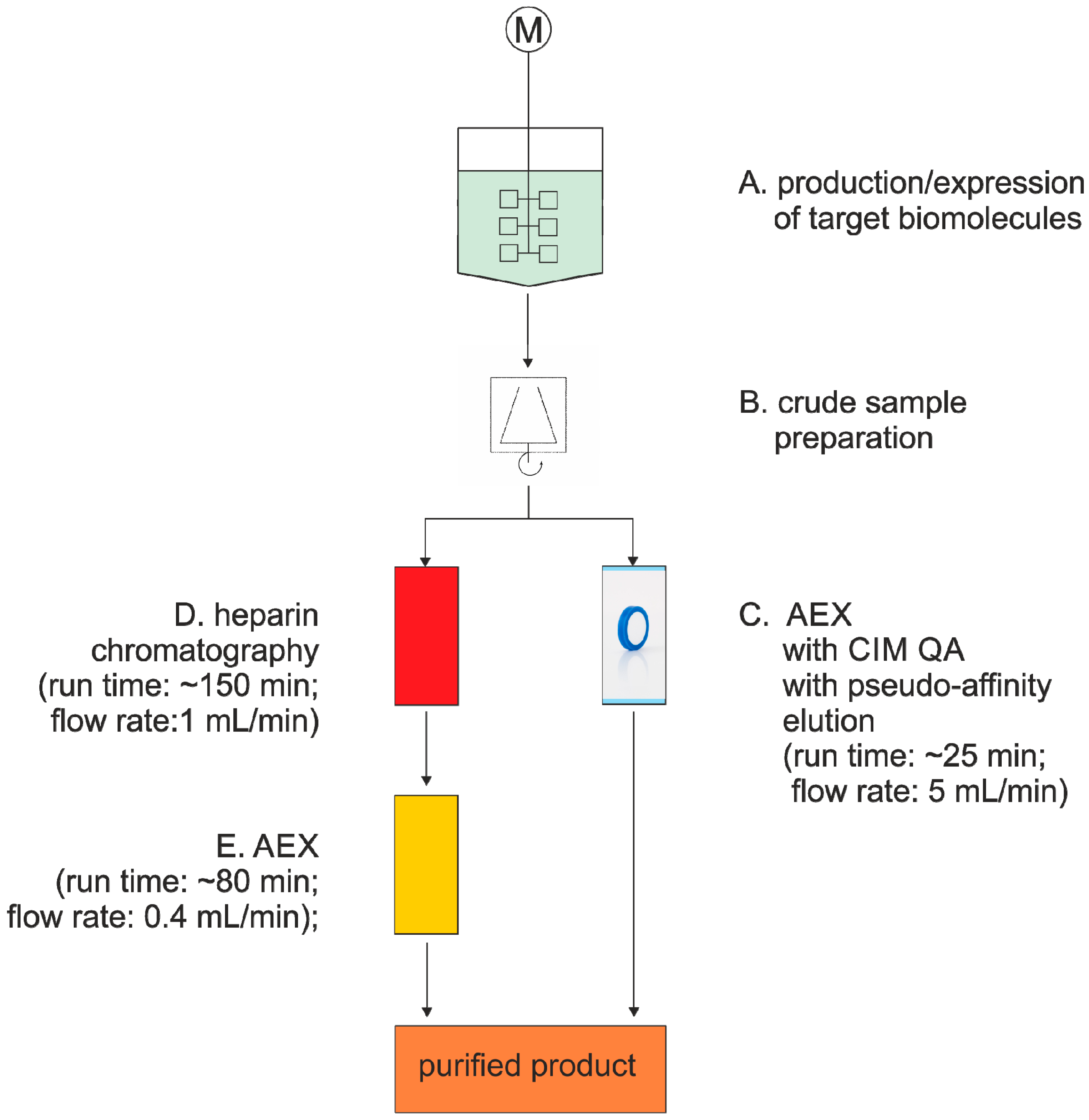
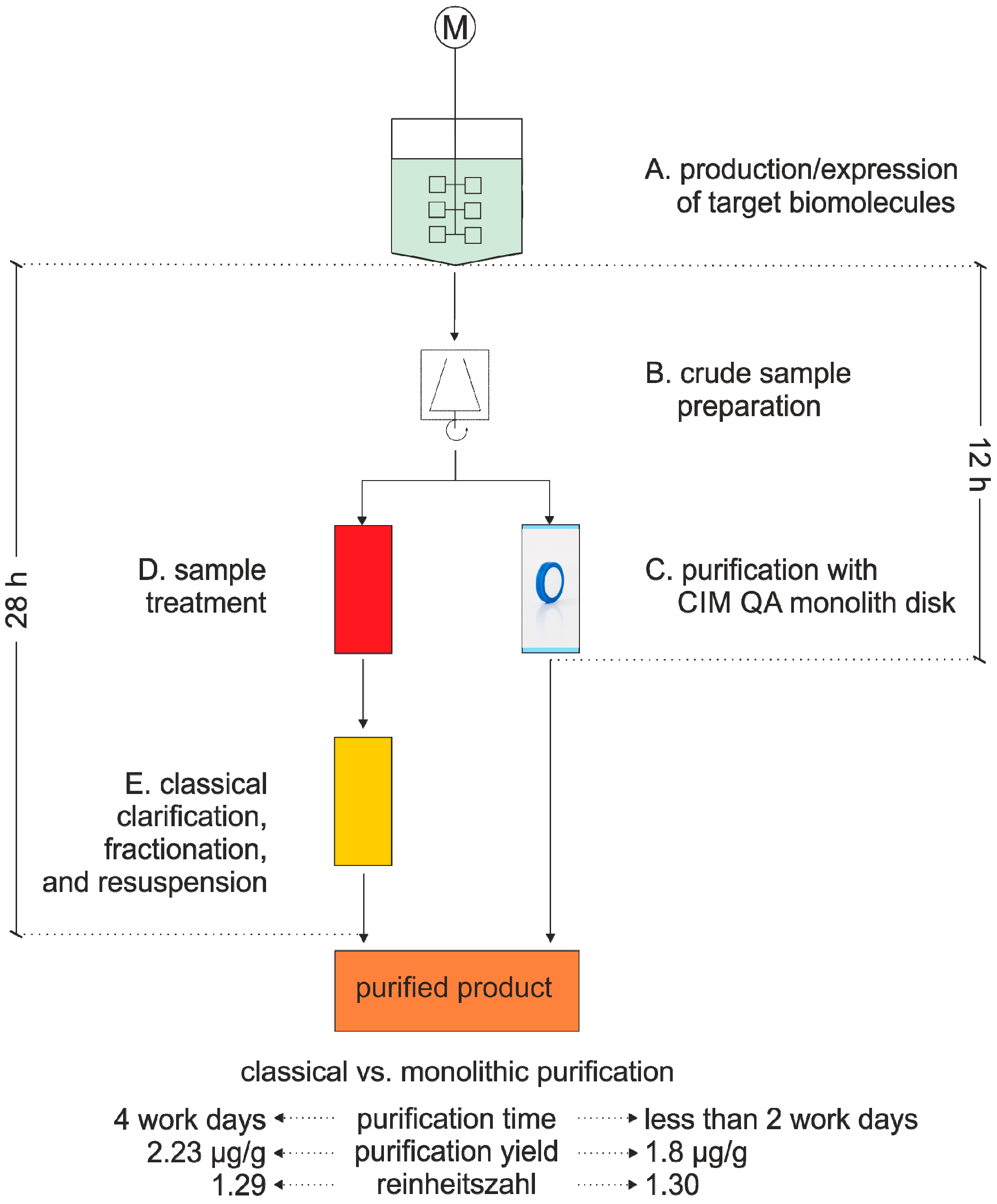
3.1. Virus Purification
| Virus | Host | Monolith | Ref. |
|---|---|---|---|
| Bacteriophage T4 | E. coli | CIM® QA | [43] |
| Flavivirus particles | Vero and baby hamster kidney (BHK) helper cells | [44] | |
| Adenovirus type 3 dodecahedric virus-like particles (VLP) | High-Five cells | CIM® analytical column (CIMac®) QA | [45] |
| Bacteriophage T7 | E. coli | Custom made CIM® QA | [46] |
| Mycobacteriophage D29 | M. smegmatis | CIM® DEAE (diethylamine) | [47] |
| Enterovirus 71 | Rhabdomyosarcoma cells | [48] | |
| Lentiviral vectors | HEK 293T cells | [49] | |
| 293T/17 cells | CIM®-IDA(iminodiacetic acid )-Ni2+ | [50] | |
| Bacteriophage VDX-10 | S. aureus | CIM® QA and CIM® DEAE | [51] |
| Bacteriophage PRD 1 | E.coli and S. enterica | [52] | |
| Burkholderia phage Phi208 | B. thailandensis | [53] | |
| Rubella virus | Human fetal lung fibroblast (MRC-5) cells | CIM® QA and CIM® SO3 | [54] |
| Influenza virus A and B | Vero cells | CIM® QA, CIM® DEAE and CIM® SO3 | [55] |
| VLPs (HBsAg) | S. cerevisiae | CIM® C4 and CIM® OH | [29] |
| Bacteriophage Phi6 | P. syringae | CIM® QA | [56] |
| Potato virus Y | Plant tissue; N. tabacum and S. tuberosum | CIM® QA | [31] |
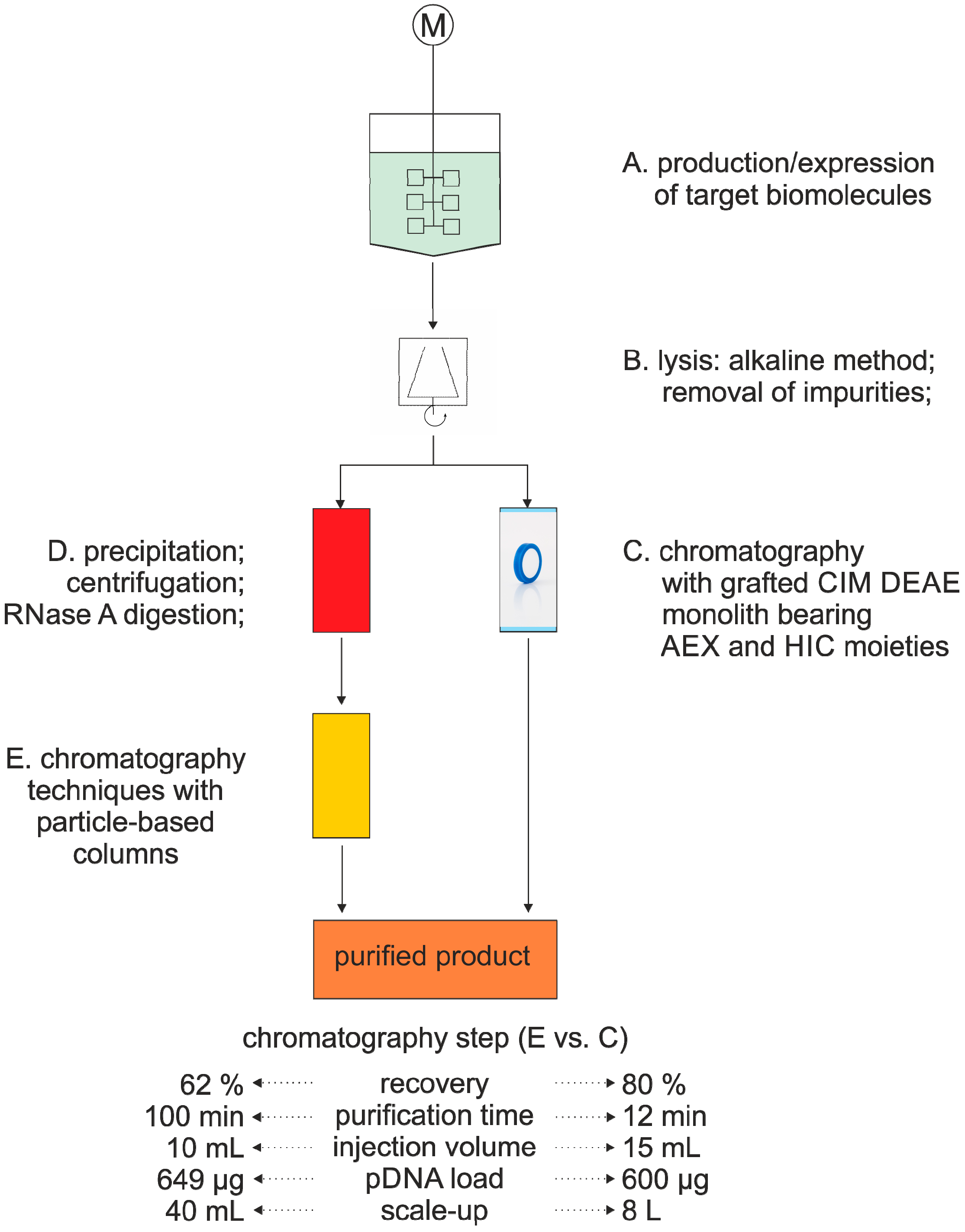
3.2. Nucleic Acid Purification
| Nucleic acid | Host | Monolith | Ref. |
|---|---|---|---|
| Plasmids | E. coli | CIM® DEAE | [70] |
| pDNA | E. coli | CIM® DEAE bearing HIC and AEX groups | [67] |
| pDNA | E. coli | CIM® DEAE, CIM® QA and CIM® C4 HLD (high ligand density) | [69] |
| pDNA HPV-16 E6/E7 | E. coli | CIMac®pDNA and Custom made CIM®-CDI (carboxydiimidazole) -arginine | [71,72] |
| scpDNA | E. coli | Custom made CIM®-CDI-histamine amino acid derivative | [73] |
| ocpDNA | E. coli | CIMac®pDNA | [74] |
| pDNA | DH5-α mutants | CIM®-CDI-Cu2+ | [75] |
| pCCIFOS-25 (ds circular DNA) | E.coli | Laboratory made poly (glycidyl methacrylate - ethylene glycol dimethacrylate) column | [76] |
| pre-miR-29 | R. sulfidophilum | Custom made CIM®-CDI-agmatine | [77] |
3.3. Protein Purification
| Protein | Host | Monolith | Ref. |
|---|---|---|---|
| TNF-α | E. coli | CIM® DEAE and CIM®-IDA-Cu2+ | [84] |
| Horseradish peroxidase | P. pastoris | CIM® DEAE | [79] |
| β-glucosidases | P. etchellsii | CIM® DEAE and CIM® EDA (ethylendiamino) | [85] |
| Host cell proteins | CHO cells | CIM® Protein-A HLD | [86] |
| IgM | IgM clone 84 | CIM® SO3, CIM® DEAE and CIM® CDI | [87] |
| proteins extracted from blood group antigens | anti-V5 hybridoma cell line | Custom made CIM® epoxy | [88] |
| IgG1 | Hybridoma cells | CIM®-IDA with four different metal ions, namely Cu2+, Ni2+, Zn2+, Co2+ | [89] |
| Factor IX | CHO cells | CIM® QA | [78] |
4. Commercially Available Monoliths
| Monolith | Principle | Manufacturer/Supplier | Ref. |
|---|---|---|---|
| CIM®, CIMmultus® and CIMac® QA | AEX | BIA separations | [19] |
| CIM®, CIMmultus® and CIMac® DEAE | |||
| CIMac® pDNA | |||
| CIMac® Adeno | |||
| CIM® and CIMac® EDA | |||
| CIM®, CIMmultus® and CIMac® SO3 | CEX | ||
| CIM® and CIMmultus® C4 A | HIC | ||
| CIM® and CIMmultus® OH | HILIC | ||
| CIM® CDI | Affinity | ||
| CIM® IDA | |||
| CIM® n-Protein A | |||
| CIM® r-Protein A | |||
| CIM® r-Protein G | |||
| CIM® r-Protein L | |||
| CIM® epoxy | |||
| ProSwift® RP monolith column | HIC | Dionex | [92] |
| ProSwift® IEX monolith column | IEX | ||
| ProSwift® ConA-1S affinity monolith column | Affinity | ||
| DNASwift® SAX-1S monolith column | AEX | ||
| PepSwift® monolith column | |||
| Onyx® monolithic C18 column | HIC | Phenomenex | [93] |
| Onyx® monolithic C8 column | |||
| Onyx® monolithic HD-C18 | |||
| Onyx® monolithic Si | |||
| Chromolith® RP-18e | |||
| Bio-Monolith® QA | AEX | Agilent technologies | [94] |
| Bio-Monolith® DEAE | |||
| Bio-Monolith® SO3 | CEX | ||
| Bio-Monolith® Protein A | Affinity | ||
| Chromolith® CapRod RP-18 endcapped 300-0.1 capillary column | HIC | Merck Millipore | [95] |
| Chromolith® Phenyl 50-4.6 HPLC column | |||
| Chromolith® FastGradient RP-18 endcapped 50-2 HPLC column | |||
| Chromolith® FastGradient RP-18 endcapped 100-3 HPLC column | |||
| Chromolith® Flash RP-18 endcapped 25-2 HPLC column | |||
| Chromolith® Flash RP-18 endcapped 25-3 HPLC column | |||
| Chromolith® Prep RP-18 endcapped 100-25 column | |||
| UNO® Monolith Anion Exchange Columns | AEX | Bio-Rad | [96] |
| UNO® Monolith Cation Exchange Columns | CEX | ||
| UNO® S1 Column | IEX | ||
| UNO® S6 Column | |||
| UNO® S12 Column | |||
| UNO® Q1 Column | |||
| UNO® Q6 Column | |||
| UNO® Q12 Column |
5. Concluding Remarks and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Center for Chemical Process Safety (CCPS). Guidelines for Process Safety in Bioprocess Manufacturing Facilities; Wiley: Hoboken, NJ, USA, 2010; p. 225. [Google Scholar]
- Kling, J. The Downstream Pinch—Upstream efficiencies, economic forces and changing technologies complicate separation and purification. BioProcess Int. 2014, 12, 1–9. [Google Scholar]
- Jungbauer, A.; Hahn, R. Polymethacrylate monoliths for preparative and industrial separation of biomolecular assemblies. J. Chromatogr. A 2008, 1184, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, A.; Savnik, A.; Jančar, J.; Krajnc, N.L. Design of monoliths through their mechanical properties. J. Chromatogr. A 2014, 1333, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, A.; Smrekar, V.; Krajnc, P.; Strancar, A. Estimation of methacrylate monolith binding capacity from pressure drop data. J. Chromatogr. A 2013, 1272, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Stulík, K.; Pacáková, V.; Suchánková, J.; Coufal, P. Monolithic organic polymeric columns for capillary liquid chromatography and electrochromatography. J. Chromatogr. B. 2006, 841, 79–87. [Google Scholar] [CrossRef]
- Tennikova, T.B.; Bleha, M.; Švec, F. High-performance membrane chromatography of proteins, a novel method of protein separation. J. Chromatogr. A 2001, 555, 97–107. [Google Scholar] [CrossRef]
- Tennikova, T.B.; Svec, F.; Belenkii, B.G. High-Performance Membrane Chromatography. A Novel Method of Protein Separation. J. Liq. Chromatogr. 1990, 13, 63–70. [Google Scholar] [CrossRef]
- Belenkii, B.G.; Podkladenko, A.M. Peculiarities of zone migration and band broadening in gradient reversed-phase high-performance liquid chromatography of proteins with respect to membrane chromatography. J. Chromatogr. A 2001, 645, 1–15. [Google Scholar] [CrossRef]
- Svec, F.; Tennikova, T.B.; Deyl, Z. Monolithic Materials: Preparation, Properties and Applications; Elsevier B. V.: Amsterdam, The Netherlands, 2003; Volume 67, pp. 1–773. [Google Scholar]
- Hilder, E.F.; Svec, F.; Fréchet, J.M. Development and application of polymeric monolithic stationary phases for capillary electrochromatography. J. Chromatogr. A 2004, 1044, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Svec, F. Recent developments in the field of monolithic stationary phases for capillary electrochromatography. J. Sep. Sci. 2005, 28, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Dubinina, N.I.; Kurenbin, O.I.; Tennikova, T.B. Peculiarities of gradient ion-exchange high-performance liquid chromatography of proteins. J. Chromatogr. A 1996, 753, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Guiochon, G. Monolithic columns in high-performance liquid chromatography. J. Chromatogr. A 2007, 1168, 101–168, discussion 100. [Google Scholar] [CrossRef] [PubMed]
- Siouffi, A.-M. Monolithic columns in high-performance liquid chromatography. J. Chromatogr. A 2006, 1126, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Vlakh, E.G.; Tennikova, T.B. Applications of polymethacrylate-based monoliths in high-performance liquid chromatography. J. Chromatogr. A 2009, 1216, 2637–2650. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P. Advances in the development of organic polymer monolithic columns and their applications in food analysis—A review. J. Chromatogr. A 2013, 1313, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Pfaunmiller, E.L.; Paulemond, M.L.; Dupper, C.M.; Hage, D.S. Affinity monolith chromatography: A review of principles and recent analytical applications. Anal. Bioanal. Chem. 2013, 405, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- BIAseparations Bia Separations. Available online: http://www.biaseparations.com/default.asp (accessed on 6 December 2014).
- Sheehan, D. Physical Biochemistry: Principles and Applications, 2nd ed.; Wiley: Oxford, UK, 2009; p. 424. [Google Scholar]
- Strancar, A.; Podgornik, A.; Barut, M.; Necina, R. Short Monolithic Columns as Stationary Phases for Biochromatography. In Modern Advances in Chromatography; Springer-Verlag: Berlin Heidelberg, Germany, 2002; pp. 49–85. [Google Scholar]
- Vlakh, E.G.; Tennikova, T.B. Preparation of methacrylate monoliths. J. Sep. Sci. 2007, 30, 2801–2813. [Google Scholar] [CrossRef] [PubMed]
- Svec, F.; Frechet, J.M.J. Kinetic Control of Pore Formation in Macroporous Polymers. Formation of “Molded” Porous Materials with High Flow Characteristics for Separations or Catalysis. Chem. Mater. 1995, 7, 707–715. [Google Scholar] [CrossRef]
- Svec, F.; Frechet, J.M.J. Temperature, a simple and efficient tool for the control of pore size distribution in macroporous polymers. Macromolecules 1995, 28, 7580–7582. [Google Scholar] [CrossRef]
- Merhar, M.; Podgornik, A.; Barut, M.; Žigon, M.; Štrancar, A. Methacrylate monoliths prepared from various hydrophobic and hydrophilic monomers—Structural and chromatographic characteristics. J. Sep. Sci. 2003, 26, 322–330. [Google Scholar] [CrossRef]
- Barut, M.; Podgornik, A.; Urbas, L.; Gabor, B.; Brne, P.; Vidic, J.; Plevcak, S.; Strancar, A. Methacrylate-based short monolithic columns: Enabling tools for rapid and efficient analyses of biomolecules and nanoparticles. J. Sep. Sci. 2008, 31, 1867–1880. [Google Scholar] [CrossRef] [PubMed]
- Benčina, M.; Podgornik, A.; Štrancar, A. Characterization of methacrylate monoliths for purification of DNA molecules. J. Sep. Sci. 2004, 27, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, N.L.; Smrekar, F.; Cerne, J.; Raspor, P.; Modic, M.; Krgovic, D.; Strancar, A.; Podgornik, A. Purification of large plasmids with methacrylate monolithic columns. J. Sep. Sci. 2009, 32, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Burden, C.S.; Jin, J.; Podgornik, A.; Bracewell, D.G. A monolith purification process for virus-like particles from yeast homogenate. J. Chromatogr. B. 2012, 880, 82–89. [Google Scholar] [CrossRef]
- Gerster, P.; Kopecky, E.-M.; Hammerschmidt, N.; Klausberger, M.; Krammer, F.; Grabherr, R.; Mersich, C.; Urbas, L.; Kramberger, P.; Paril, T.; et al. Purification of infective baculoviruses by monoliths. J. Chromatogr. A 2013, 1290, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Rupar, M.; Ravnikar, M.; Tušek-Žnidarič, M.; Kramberger, P.; Glais, L.; Gutiérrez-Aguirre, I. Fast purification of the filamentous Potato virus Y using monolithic chromatographic supports. J. Chromatogr. A 2013, 1272, 33–40. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, S.; Wätzig, H. Performance comparison between monolithic C18 and conventional C18 particle-packed columns in the liquid chromatographic determination of propranolol HCl. Turkish J. Chem. 2006, 30, 543–552. [Google Scholar]
- Szumski, M.; Buszewski, B. Preparation of Monolithic Capillary Chromatographic Columns Using Supercritical Fluid as a Porogen Solvent. Chromatographia 2014, 77, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Liu, Z.; Wang, H.; Lin, H.; Dong, J.; Zou, H. Recent development of hybrid organic-silica monolithic columns in CEC and capillary LC. Electrophoresis 2015, 36, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Zheng, M.-M.; Feng, Y.-Q. Preparation of organic-inorganic hybrid silica monolith with octyl and sulfonic acid groups for capillary electrochromatograhpy and application in determination of theophylline and caffeine in beverage. J. Chromatogr. A 2010, 1217, 3547–3556. [Google Scholar] [CrossRef] [PubMed]
- Mayadunne, E.; El Rassi, Z. Facile preparation of octadecyl monoliths with incorporated carbon nanotubes and neutral monoliths with coated carbon nanotubes stationary phases for HPLC of small and large molecules by hydrophobic and π-π interactions. Talanta 2014, 129, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Peyrin, E.; Caron, C.; Garrel, C.; Ravel, A.; Villet, A.; Grosset, C.; Favier, A. DNA migration regimes in hydrodynamic chromatography and slalom chromatography: Evidence for a transition. 2001, 55, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.X.; Courderot, C.; Truong, T.; Pierre, J.; Thomassin, M.; Guinchard, C.; Guillaume, Y.C.; Nicod, L. Supercoiled circular DNA and protein retention in non-equilibrium chromatography Temperature and velocity dependence: Testimony of a transition. J. Chromatogr. A 2002, 950, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Jandera, P.; Schoenmakers, P. Preparation of monolithic columns with target mesopore-size distribution for potential use in size-exclusion chromatography. J. Chromatogr. A 2007, 1150, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-C. Baculovirus Vectors for Gene Therapy. Adv. Virus Res. 2006, 68, 287–320. [Google Scholar] [PubMed]
- Wang, C.-Y.; Li, F.; Yang, Y.; Guo, H.-Y.; Wu, C.-X.; Wang, S. Recombinant baculovirus containing the diphtheria toxin A gene for malignant glioma therapy. Cancer Res. 2006, 66, 5798–5806. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Y.; Chen, C.-Y.; Chang, M.D.-T.; Matsuura, Y.; Hu, Y.-C. Concanavalin A affinity chromatography for efficient baculovirus purification. Biotechnol. Prog. 2009, 25, 1669–1677. [Google Scholar] [PubMed]
- Smrekar, F.; Ciringer, M.; Peterka, M.; Podgornik, A. Strancar, a Purification and concentration of bacteriophage T4 using monolithic chromatographic supports. J. Chromatogr. B 2008, 861, 177–180. [Google Scholar] [CrossRef]
- Mundle, S.T.; Giel-Moloney, M.; Kleanthous, H.; Pugachev, K.V.; Anderson, S.F. Preparation of pure, high titer, pseudoinfectious Flavivirus particles by hollow fiber tangential flow filtration and anion exchange chromatography. Vaccine 2014, in press. [Google Scholar]
- Urbas, L.; Jarc, B.L.; Barut, M.; Zochowska, M.; Chroboczek, J.; Pihlar, B.; Szolajska, E. Purification of recombinant adenovirus type 3 dodecahedric virus-like particles for biomedical applications using short monolithic columns. J. Chromatogr. A 2011, 1218, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Smrekar, F.; Ciringer, M.; Strancar, A.; Podgornik, A. Characterisation of methacrylate monoliths for bacteriophage purification. J. Chromatogr. A 2011, 1218, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wen, Z.; Li, N.; Yang, W.; Hu, L.; Wang, J.; Yin, Z.; Dong, X.; Li, J. Purification and concentration of mycobacteriophage D29 using monolithic chromatographic columns. J. Virol. Methods 2012, 186, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kattur Venkatachalam, A.R.; Szyporta, M.; Kiener, T.K.; Balraj, P.; Kwang, J. Concentration and purification of enterovirus 71 using a weak anion-exchange monolithic column. Virol. J. 2014, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, V.; Peixoto, C.; Rodrigues, A.F.; Cruz, P.E.; Alves, P.M.; Coroadinha, A.S.; Carrondo, M.J.T. Downstream processing of lentiviral vectors: Releasing bottlenecks. Hum. Gene Ther. Methods 2012, 23, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cheeks, M.C.; Kamal, N.; Sorrell, A.; Darling, D.; Farzaneh, F.; Slater, N.K.H. Immobilized metal affinity chromatography of histidine-tagged lentiviral vectors using monolithic adsorbents. J. Chromatogr. A 2009, 1216, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Kramberger, P.; Honour, R.C.; Herman, R.E.; Smrekar, F.; Peterka, M. Purification of the Staphylococcus aureus bacteriophages VDX-10 on methacrylate monoliths. J. Virol. Methods 2010, 166, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, H.M.; Domanska, A.; Bamford, D.H. Monolithic ion exchange chromatographic methods for virus purification. Virology 2012, 434, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Lehman, S.M.; Vandersteegen, K.; Vandenheuvel, D.; Philippe, D.L.; Cornelissen, A.; Clokie, M.R.J.; García, A.J.; De Proft, M.; Maes, M.; et al. CIM(®) monolithic anion-exchange chromatography as a useful alternative to CsCl gradient purification of bacteriophage particles. Virology 2012, 434, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Forcic, D.; Brgles, M.; Ivancic-Jelecki, J.; Santak, M.; Halassy, B.; Barut, M.; Jug, R.; Markušić, M.; Strancar, A. Concentration and purification of rubella virus using monolithic chromatographic support. J. Chromatogr. B 2011, 879, 981–986. [Google Scholar] [CrossRef]
- Banjac, M.; Roethl, E.; Gelhart, F.; Kramberger, P.; Jarc, B.L.; Jarc, M.; Strancar, A.; Muster, T.; Peterka, M. Purification of Vero cell derived live replication deficient influenza A and B virus by ion exchange monolith chromatography. Vaccine 2014, 32, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Romanovskaya, A.; Sarin, L.P.; Bamford, D.H.; Poranen, M.M. High-throughput purification of double-stranded RNA molecules using convective interaction media monolithic anion exchange columns. J. Chromatogr. A 2013, 1278, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgener, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.D.; Rhodes, D.G.; Burgess, D.J. DNA-based therapeutics and DNA delivery systems: A comprehensive review. AAPS J. 2005, 7, E61–E77. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Germán, P.; Prazeres, D.M.F.; Guzmán, R.; Montesinos-Cisneros, R.M.; Tejeda-Mansir, A. Purification of plasmid DNA using tangential flow filtration and tandem anion-exchange membrane chromatography. Bioprocess Biosyst. Eng. 2009, 32, 615–623. [Google Scholar] [CrossRef]
- Eon-Duval, A.; MacDuff, R.H.; Fisher, C.A.; Harris, M.J.; Brook, C. Removal of RNA impurities by tangential flow filtration in an RNase-free plasmid DNA purification process. Anal. Biochem. 2003, 316, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kahn, D.W.; Butler, M.D.; Cohen, D.L.; Gordon, M.; Kahn, J.W.; Winkler, M.E. Purification of plasmid DNA by tangential flow filtration. Biotechnol. Bioeng. 2000, 69, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ruckenstein, E. Membrane Chromatography: Preparation and Applications to Protein Separation. Biotechnol. Prog. 1999, 15, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Kendall, D.; Lye, G.J.; Levy, M.S. Purification of plasmid DNA by an integrated operation comprising tangential flow filtration and nitrocellulose adsorption. Biotechnol. Bioeng. 2002, 79, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Endres, H.N.; Johnson, J.A.; Ross, C.A.; Welp, J.K.; Etzel, M.R. Evaluation of an ion-exchange membrane for the purification of plasmid DNA. Biotechnol. Appl. Biochem. 2003, 37, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, D.M.F.; Schluep, T.; Cooney, C. Preparative purification of supercoiled plasmid DNA using anion-exchange chromatography. J. Chromatogr. A 1998, 806, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Diogo, M.M.; Queiroz, J.A.; Monteiro, G.A.; Martins, S.A.; Ferreira, G.N.; Prazeres, D.M. Purification of a cystic fibrosis plasmid vector for gene therapy using hydrophobic interaction chromatography. Biotechnol. Bioeng. 2000, 68, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Smrekar, V.; Smrekar, F.; Strancar, A.; Podgornik, A. Single step plasmid DNA purification using methacrylate monolith bearing combination of ion-exchange and hydrophobic groups. J. Chromatogr. A 2013, 1276, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Peterka, M.; Glover, D.; Kramberger, P.; Banjac, M.; Podgornik, A.; Barut, M.; Štrancar, A. Short Monolithic Columns—An Enabling Technology for the Purification of Proteins, DNA, and Viruses. Bioprocess. J. 2005, 4, 79–84. [Google Scholar]
- Smrekar, F.; Podgornik, A.; Ciringer, M.; Kontrec, S.; Raspor, P.; Strancar, A.; Peterka, M. Preparation of pharmaceutical-grade plasmid DNA using methacrylate monolithic columns. Vaccine 2010, 28, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, N.L.; Smrekar, F.; Strancar, A.; Podgornik, A. Adsorption behavior of large plasmids on the anion-exchange methacrylate monolithic columns. J. Chromatogr. A 2011, 1218, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.M.; Queiroz, J.A.; Sousa, F.; Sousa, A. Optimization of supercoiled HPV-16 E6/E7 plasmid DNA purification with arginine monolith using design of experiments. J. Chromatogr. B 2014, 978-979C, 145–150. [Google Scholar]
- Soares, A.; Queiroz, J.A.; Sousa, F.; Sousa, A. Purification of human papillomavirus 16 E6/E7 plasmid deoxyribonucleic acid-based vaccine using an arginine modified monolithic support. J. Chromatogr. A 2013, 1320, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Almeida, A.M.; Černigoj, U.; Sousa, F.; Queiroz, J.A. Histamine monolith versatility to purify supercoiled plasmid deoxyribonucleic acid from Escherichia coli lysate. J. Chromatogr. A 2014, 1355, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gabor, B.; Černigoj, U.; Barut, M.; Štrancar, A. Reversible entrapment of plasmid deoxyribonucleic acid on different chromatographic supports. J. Chromatogr. A 2013, 1311, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.J.; Tan, L.; Jeong, M.H.; Kim, J.-H.; Choe, W.-S. Monolith-based immobilized metal affinity chromatography increases production efficiency for plasmid DNA purification. J. Chromatogr. A 2011, 1218, 5273–5278. [Google Scholar] [CrossRef] [PubMed]
- Ongkudon, C.M.; Pan, S.; Danquah, M.K. An innovative monolithic column preparation for the isolation of 25 kilo base pairs DNA. J. Chromatogr. A 2013, 1318, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Sousa, A.; Queiroz, J.A.; Figueiras, A.; Sousa, F. Pharmaceutical-grade pre-miR-29 purification using an agmatine monolithic support. J. Chromatogr. A 2014, 1368, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Passos, D.F.; Ferraz, H.C.; Castilho, L.R. Anion-exchange purification of recombinant factor IX from cell culture supernatant using different chromatography supports. J. Chromatogr. B 2013, 938, 111–118. [Google Scholar] [CrossRef]
- Krainer, F.W.; Pletzenauer, R.; Rossetti, L.; Herwig, C.; Glieder, A.; Spadiut, O. Purification and basic biochemical characterization of 19 recombinant plant peroxidase isoenzymes produced in Pichia pastoris. Protein Expr. Purif. 2014, 95, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hemström, P.; Nordborg, A.; Irgum, K.; Svec, F.; Fréchet, J.M.J. Polymer-based monolithic microcolumns for hydrophobic interaction chromatography of proteins. J. Sep. Sci. 2006, 29, 25–32. [Google Scholar] [CrossRef]
- Chen, M.-L.; Li, L.-M.; Yuan, B.-F.; Ma, Q.; Feng, Y.-Q. Preparation and characterization of methacrylate-based monolith for capillary hydrophilic interaction chromatography. J. Chromatogr. A 2012, 1230, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Leblebici, P.; Leblebici, M.E.; Ferreira-da-Silva, F.; Rodrigues, A.E.; Pais, L.S. Separation of human immunoglobulin G subclasses on a protein A monolith column. J. Chromatogr. B 2014, 962, 89–93. [Google Scholar] [CrossRef]
- Nikolayenko, I.V.; Galkin, O.Y.; Grabchenko, N.I.; Spivak, M.Y. Preparation of highly purified human IgG, IgM, and IgA for immunization and immunoanalysis. Ukr. Bioorg. Acta 2005, 2, 3–11. [Google Scholar]
- Sushma, K.; Vijayalakshmi, M.A.; Krishnan, V.; Satheeshkumar, P.K. Cloning, expression, purification and characterization of a single chain variable fragment specific to tumor necrosis factor alpha in Escherichia coli. J. Biotechnol. 2010, 156, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, R.K.; Mishra, S.; Vijayalakshmi, M.A. Purification of ß-glucosidases from Pichia etchellsii using CIM monolith columns. Appl. Biochem. Biotechnol. 2011, 164, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Tscheliessnig, A.; Jungbauer, A. High-performance monolith affinity chromatography for fast quantitation of immunoglobulin G. J. Chromatogr. A 2009, 1216, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.T.; Lee, J.; Latiff, S.M.A.; Chuah, C.; Toh, P.; Lee, W.Y.; Gagnon, P. Characterization and removal of aggregates formed by nonspecific interaction of IgM monoclonal antibodies with chromatin catabolites during cell culture production. J. Chromatogr. A 2013, 1291, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Mönster, A.; Hiller, O.; Grüger, D.; Blasczyk, R.; Kasper, C. Isolation and purification of blood group antigens using immuno-affinity chromatography on short monolithic columns. J. Chromatogr. A 2011, 1218, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Rajak, P.; Vijayalakshmi, M.A.; Jayaprakash, N.S. Purification of monoclonal antibodies, IgG1, from cell culture supernatant by use of metal chelate convective interaction media monolithic columns. Biomed. Chromatogr. 2012, 26, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.; Gil, G.C.; Cadiz, A.; Velander, W.H.; Zhang, C.; Van Cott, K.E. Purification of recombinant DNA-derived factor IX produced in transgenic pig milk and fractionation of active and inactive subpopulations. J. Chromatogr. A 2004, 1026, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Endicott, E. Agilent Technologies, BIA Separations GmbH Sign Agreement Giving Agilent Access to BIA Separations’ Bio-Monolithic Technology. Available online: http://www.agilent.com/about/newsroom/presrel/2008/29may-ca08038.html (accessed on 22 March 2015).
- Dionex. Available online: http://www.dionex.com/en-us/products/columns/bio/protein/proswift-iex/lp-73379.html (accessed on 15 April 2015).
- Onyx HPLC information. Available online: https://www.phenomenex.com/onyx (accessed on 6 December 2014).
- Bio-Monolith - Agilent. Available online: http://www.chem.agilent.com/en-US/products-services/Columns-Sample-Preparation/LC-LC-MS-Columns/BioHPLC/Bio-Monolith/Pages/default.aspx (accessed on 30 January 2015).
- Chromolith HPLC columns. Available online: http://www.merckmillipore.com/INTL/en/analytics-sample-prep/chromatography-for-analysis/analytical-hplc/chromolith-hplc-columns/Rk2b.qB.cMMAAAE_hPB3.Lxi,nav (accessed on 6 December 2014).
- Chromatography Columns - UNO monolithic columns. Available online: http://www.bio-rad.com/de-at/category/chromatography-columns (accessed on 30 January 2015).
- Smrekar, F.; Ciringer, M.; Jančar, J.; Raspor, P.; Strancar, A.; Podgornik, A. Optimization of lytic phage manufacturing in bioreactor using monolithic supports. J. Sep. Sci. 2011, 34, 2152–2158. [Google Scholar] [PubMed]
- Fee, C.; Nawada, S.; Dimartino, S. 3D printed porous media columns with fine control of column packing morphology. J. Chromatogr. A 2014, 1333, 18–24. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajamanickam, V.; Herwig, C.; Spadiut, O. Monoliths in Bioprocess Technology. Chromatography 2015, 2, 195-212. https://doi.org/10.3390/chromatography2020195
Rajamanickam V, Herwig C, Spadiut O. Monoliths in Bioprocess Technology. Chromatography. 2015; 2(2):195-212. https://doi.org/10.3390/chromatography2020195
Chicago/Turabian StyleRajamanickam, Vignesh, Christoph Herwig, and Oliver Spadiut. 2015. "Monoliths in Bioprocess Technology" Chromatography 2, no. 2: 195-212. https://doi.org/10.3390/chromatography2020195