Quantification of Carbonyl Compounds Generated from Ozone-Based Food Colorants Decomposition Using On-Fiber Derivatization-SPME-GC-MS
Abstract
:1. Introduction
| Study | Matrix | Analysis Method | Internal Standard | (Range of Carbon Number of) Target Carbonyls | (Range of) LOD/ppb(v) | Description of the Study |
|---|---|---|---|---|---|---|
| Beránek et al., 2008 [26] | Aqueous particulate matter extracts of wood smoke and diesel exhaust | GC-MS (TIC) | butanal-d2 and benzaldehyde-d6 | C1-C12 | 0.1–55 | A SPME-GC-MS method for trace analysis of a wide range of carbonyls was developed. Headspace and direct extractions from liquid phase with on-fiber derivatization were tested. Effects of extraction time and temperature were tested. |
| Ethanal | 3.7 | |||||
| Benzaldehyde | 2.3 | |||||
| 2-Furfural | 35 | |||||
| Hexanal | 0.7 | |||||
| Saison et al., 2008 [27] | Beer | GC-MS (TIC) | 2-heptanol and guaiacol or p-fluorobenzaldehyde | C3-C10 | 0.003–310 | Two SPME-GC-MS procedures for analysis of volatiles in beer were developed. Effects of time and temperature of PFBHA doping and derivatization, salt addition, and matrix effects were studied. |
| Hexanal | 0.028 | |||||
| Benzaldehyde | 0.078 | |||||
| 2-Furfural | 6.0 | |||||
| Trenholm et al., 2008 [32] | Municipal wastewater | GC-MS (TIC/SIM) | acetone-d6 | C1 | 3.7 | A rapid and automated SPME-GC-MS method utilizing SPME autosampler was developed for formaldehyde analysis. Reproducibility and background contamination were reported. |
| Tsai et al., 2003 [34] | Water (bi-distilled water, well water, chlorinated tap water) | GC-MS-(TIC) | decafluorobiphenyl | C1-C5 | 0.12–0.34 | A SPME-GC-MS method for the analysis of short chain aliphatic aldehydes was developed. Effects of time for PFBHA doping and derivatization were tested. |
| Deng et al., 2004 [23] | Human blood | GC-MS (TIC/SIM) | None | C2-C7 | 0.44 × 10−4–6.0 × 10−4 | A SPME-GC-MS method was developed for the analysis of short chain aliphatic aldehydes in human blood as a diagnostic measure of cancer status. Effects of derivatization time and temperature were tested. |
| Ethanal | 0.44 × 10−4 | |||||
| Hexanal | 6.0 × 10−4 | |||||
| Wang et al., 2005 [33] | Particle board, wine and fish | GC-MS/FID | None | C1-C9 | 2–25 | An automated SPME-GC-MS method was developed for the analysis of low-molecular mass aldehydes in the given sample. PFPH was compared with PFBHA. Limiting factor was determined to be diffusion of analytes from sample to headspace. |
| Ethanal | 0.5 | |||||
| Hexanal | 0.5 | |||||
| This study | Wastewater (ozonated fruit leather) solution / suspension) | GC-MS (TIC/SIM) | None | C2-C7 | 0.016–0.030 | A SPME-GC-MS method was developed for the analysis of carbonyls from ozone-based food colorant decomposition. Effects of dopant concentration, derivatization temperature and time were studied. Inaccuracy from competitive derivatization was minimized with a short derivatization time. |
| Ethanal | 0.030 | |||||
| Hexanal | 0.029 | |||||
| Benzaldehyde | 0.016 | |||||
| 2-Furfural | 0.016 |
2. Experimental Section
2.1. Reagents and Materials
2.2. Response Factor Calculation
2.3. On-fiber Derivatization HS-SPME
2.4. GC-MS Condition for Carbonyls Analysis
2.5. Validation of the Method
2.6. Analyses of Ozonated Fruit Leather Solution / Suspension Samples
3. Results and Discussion
3.1. Doping of PFBHA
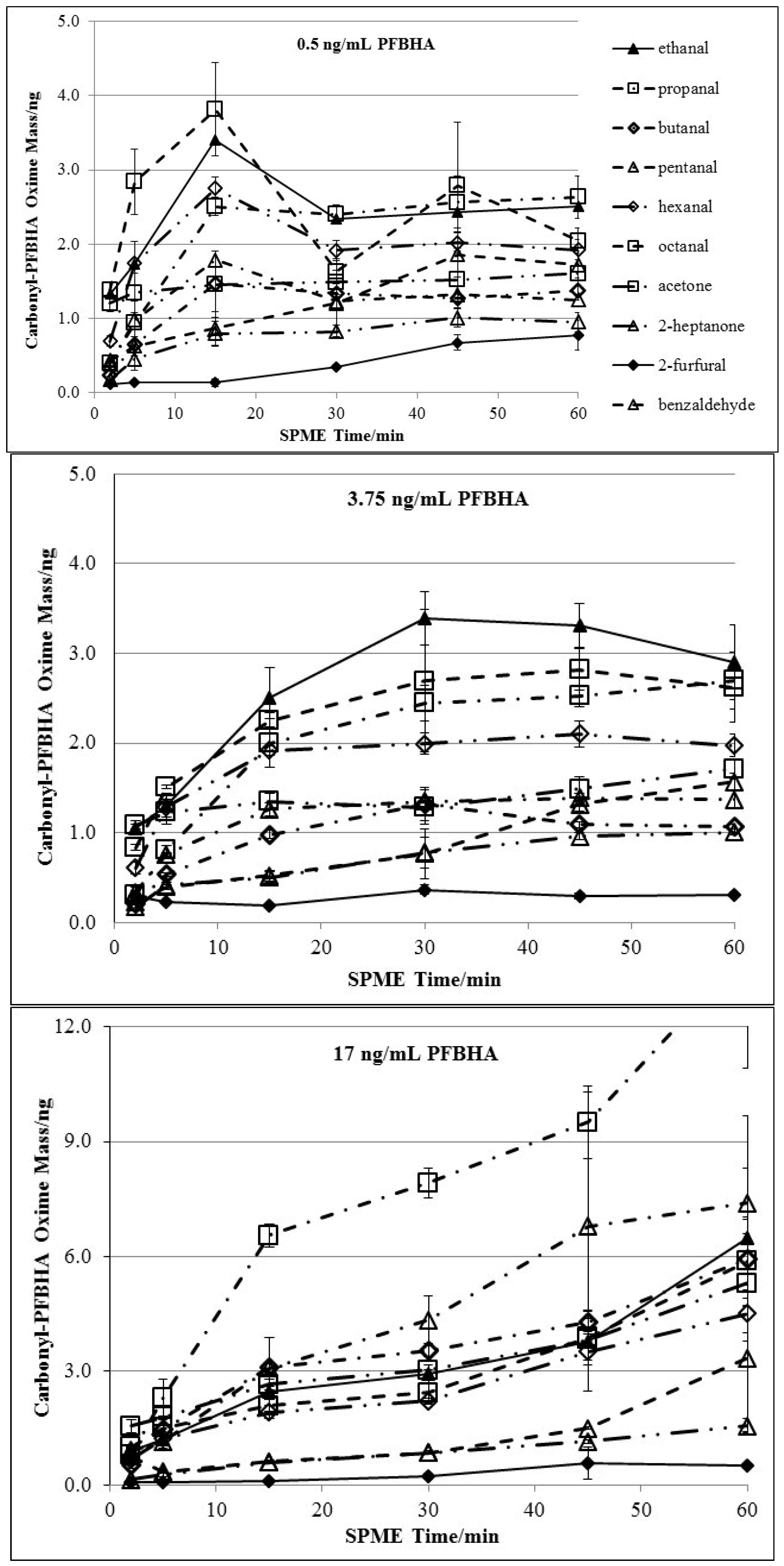
| Chemical | 2 min derivatization | 5 min derivatization | 15 min derivatization | |||
|---|---|---|---|---|---|---|
| P value | Significant Difference | P value | Significant Difference | P value | Significant Difference | |
| Ethanal | 0.011 | N | 0.052 | N | 0.003 | Y |
| Propanal | 0.003 | Y | 0.003 | Y | 0.000 | Y |
| Butanal | 0.001 | Y | 0.001 | Y | 0.000 | Y |
| Pentanal | 0.000 | Y | 0.084 | N | 0.008 | Y |
| Hexanal | 0.399 | N | 0.021 | N | 0.000 | Y |
| Octanal | 0.001 | Y | 0.002 | Y | 0.002 | Y |
| Acetone | 0.016 | N | 0.018 | N | 0.002 | Y |
| 2-Heptanone | 0.671 | N | 0.180 | N | 0.036 | N |
| 2-Furfural | 0.015 | N | 0.000 | Y | 0.057 | N |
| Benzaldehyde | 0.001 | Y | 0.013 | N | 0.050 | N |
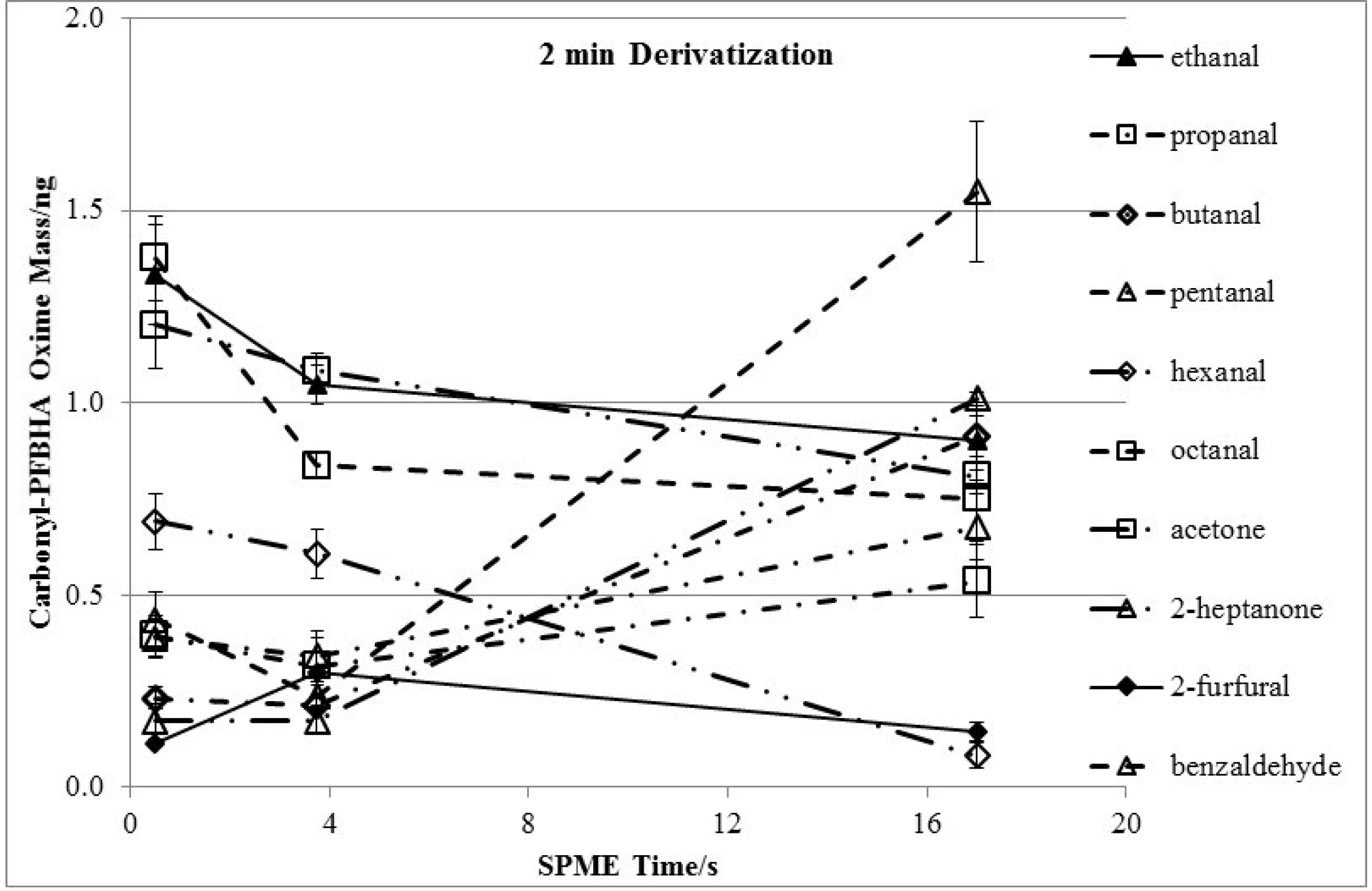
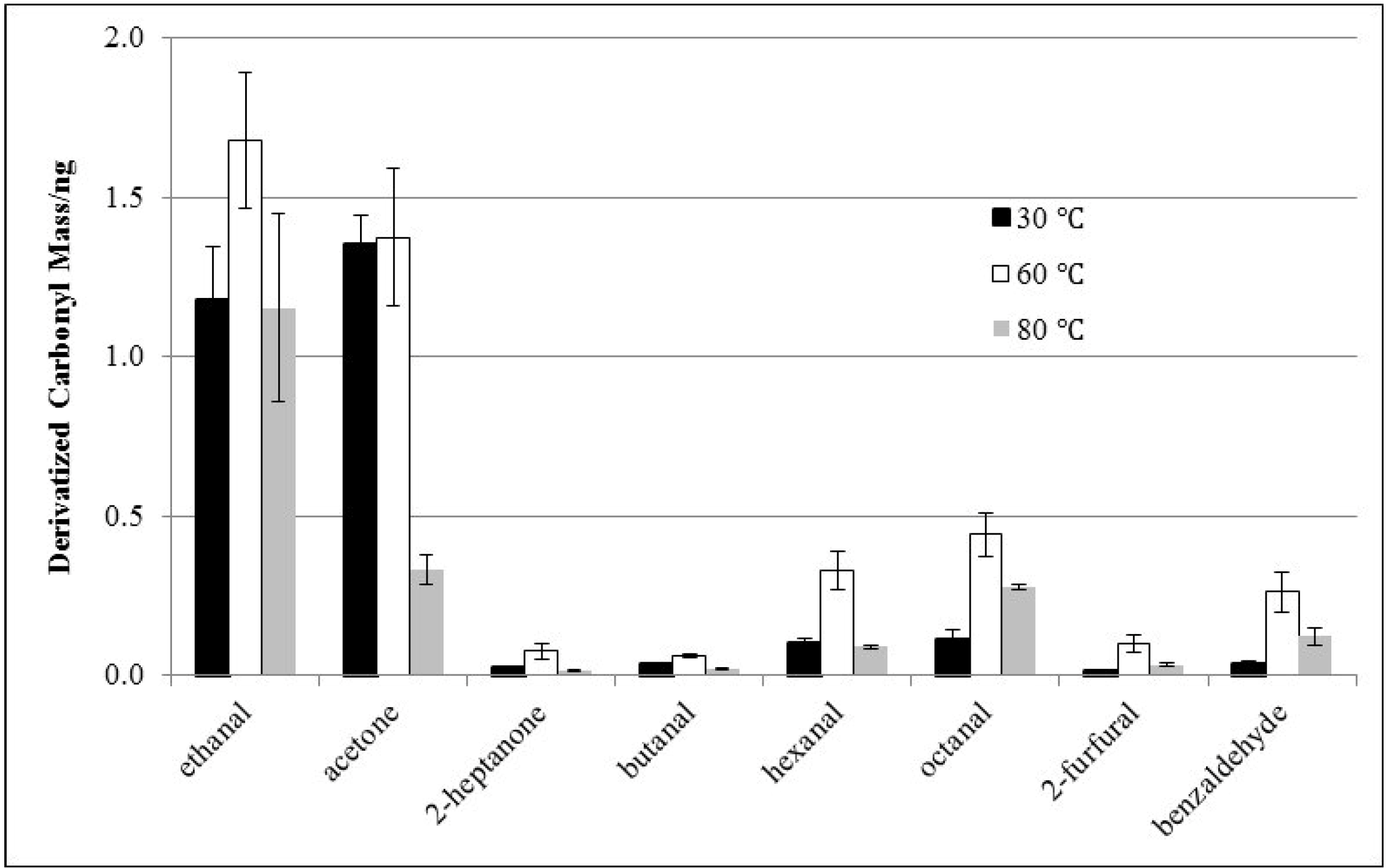
3.2. Effects of Derivatization Temperature
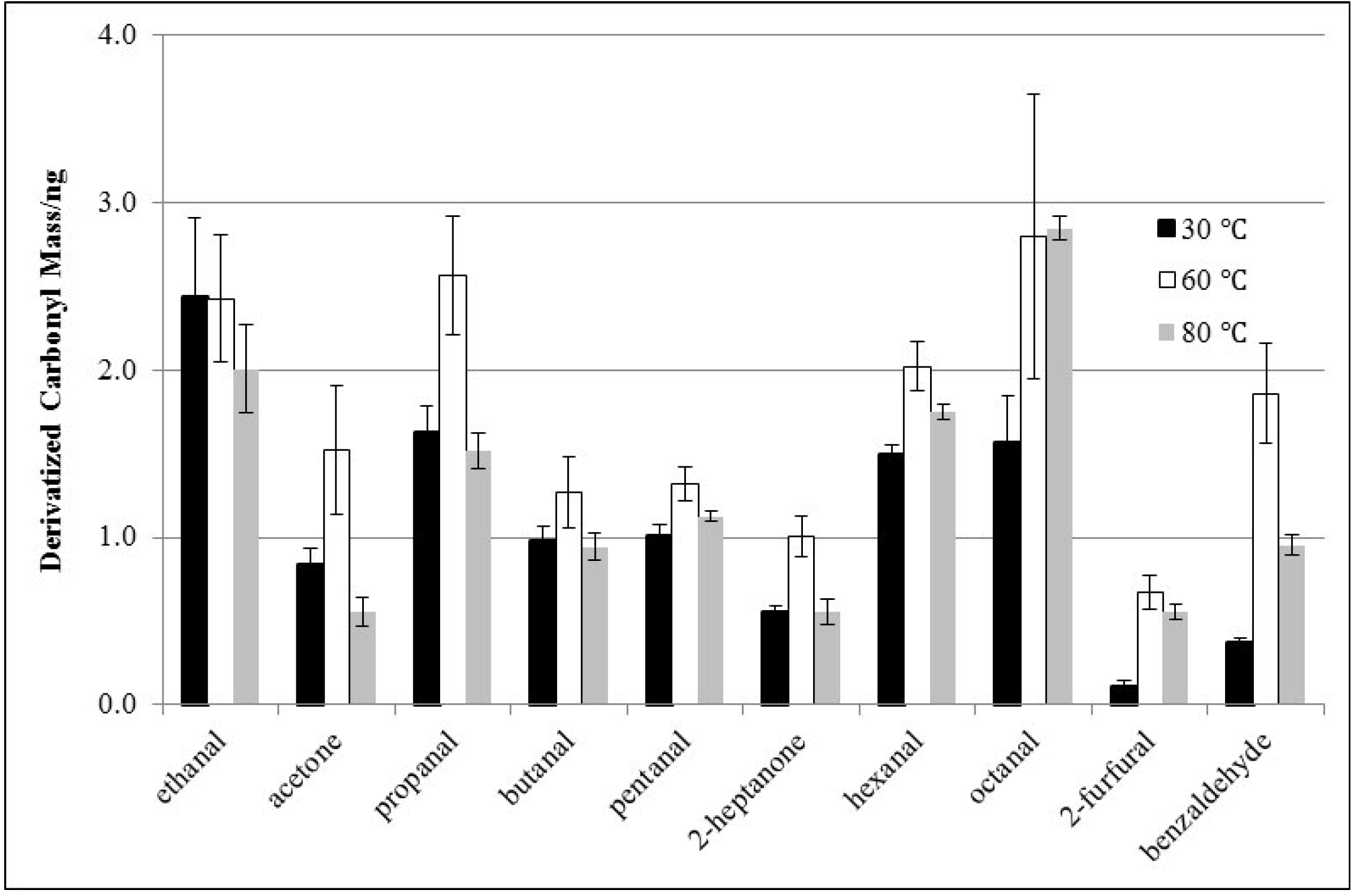
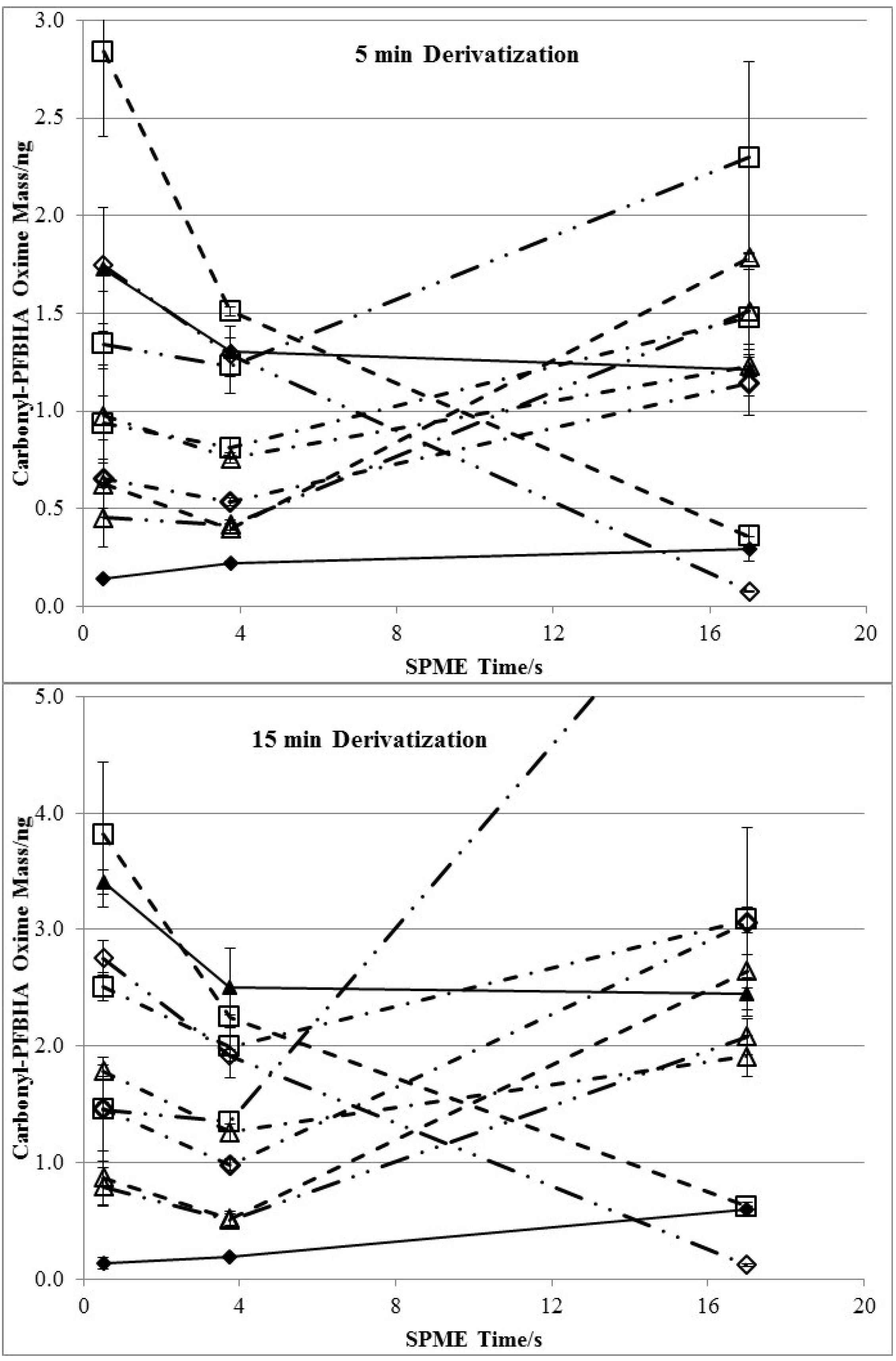
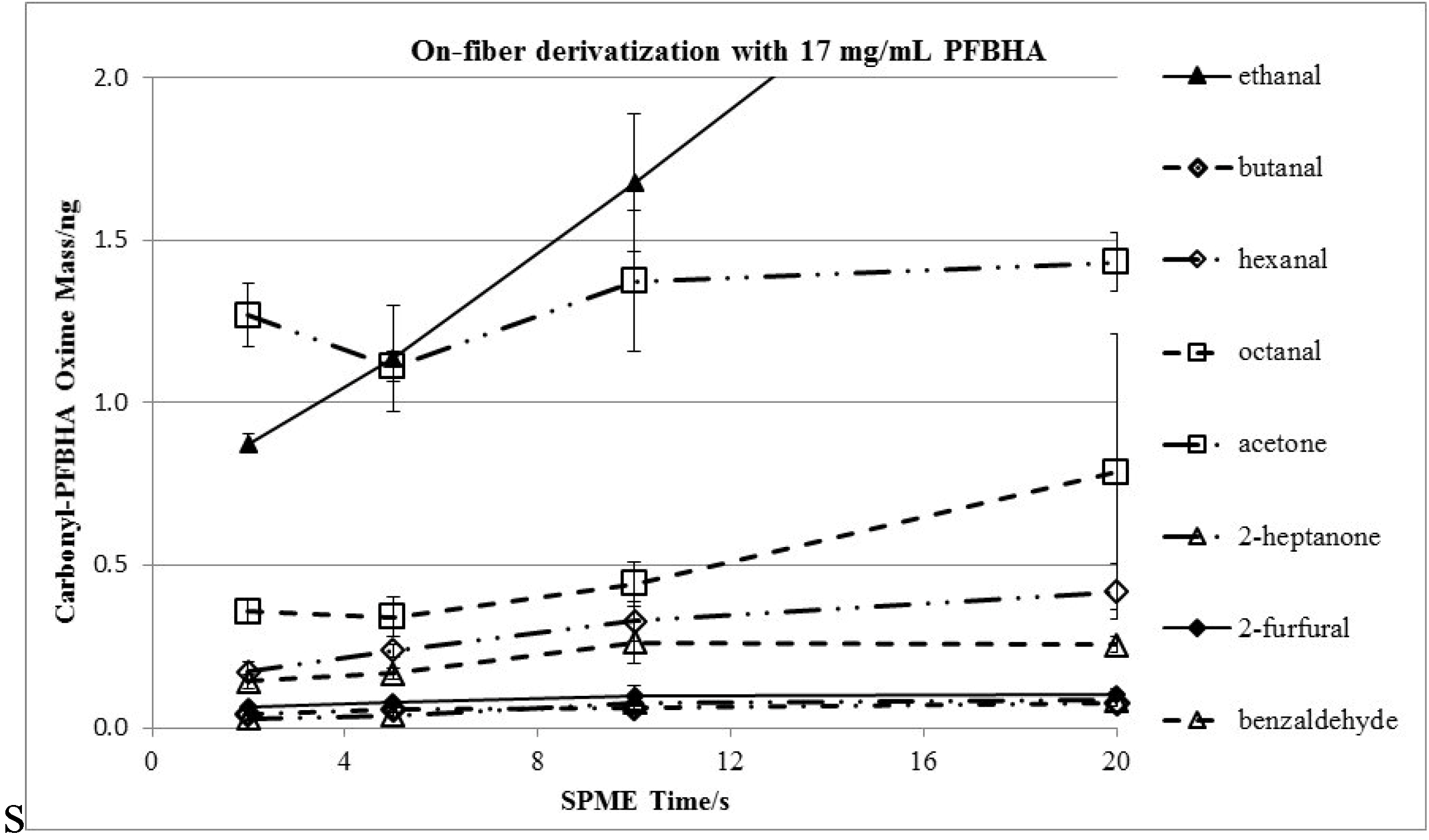
3.3. Effects of Derivatization Time and Displacement of 2-Furfural
| Chemical | Henry’s Law Constant/(M/atm) | RF | Extraction-time Linearity (R2) | Average Uptake of Carbonyls/ng e | Average RSD (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2–60 min | 2–20 s | 60 min | 5 s | 2–60 min | 2–20 s | 5 s | |||
| Ethanal | 15 a | 86,600 | 0.932 | 0.932 | 6.49 ± 1.82 | 1.14 ± 0.16 f | 12.7 | 10.2 | 14.4 |
| Propanal | 13 a | 60,200 | 0.942 | ND | 14.01 ± 3.10 | ND | 13.7 | ND | ND |
| Butanal | 8.7 a | 342,000 | 0.930 | 0.969 | 5.93 ± 1.02 | 0.05 ± 0.01 | 11.1 | 8.94 | 10.4 |
| Pentanal | 6.8 a | 73.40 | 0.973 | ND | 7.39 ± 2.27 | ND | 24.6 | ND | ND |
| Hexanal | 4.7 a | 64,200 | 0.973 | 0.957 | 4.50 ± 0.74 | 0.24 ± 0.02 | 12.3 | 15.9 | 7.90 |
| Octanal | 1.9 a | 31,400 | 0.941 | 0.930 | 5.87 ± 1.15 | 0.34 ± 0.06 | 10.5 | 24.4 | 17.7 |
| Acetone | 25 a | 112,000 | 0.968 | 0.562 | 5.29 ± 1.29 | 1.11 ± 0.05 | 8.64 | 8.43 | 4.19 |
| 2-Heptanone | 7.0 a | 125,300 | 0.988 | 0.825 | 1.56 ± 0.18 | 0.04 ± 0.01 | 10.8 | 25.0 | 17.3 |
| 2-Furfural | 263.2 b | 100,800 | 0.874 | 0.963 d | 0.51 ± 0.05 | 0.08 ± 0.00 | 29.1 | 14.3 | 1.61 |
| Benzaldehyde | 42 c | 130,900 | 0.776 | 0.999 d | 3.32 ± 1.94 | 0.17 ± 0.02 | 17.1 | 14.4 | 9.34 |
3.4. Method Validation
| Compound | Calibration Curve | R2 | LOD (µg/L) |
|---|---|---|---|
| Ethanal | y = 1.2 × 103x + 70324 | 0.9831 | 0.030 |
| Hexanal | y = 9.2 × 102x + 29024 | 0.9959 | 0.029 |
| 2-Furfural * | y = 3.7 × 104x + 35313 | 0.9579 | 0.016 |
| Benzaldehyde | y = 4.1 × 104x + 38523 | 0.9874 | 0.016 |

3.5. Ozonated Fruit Leather Solution/Suspension Sample Analysis
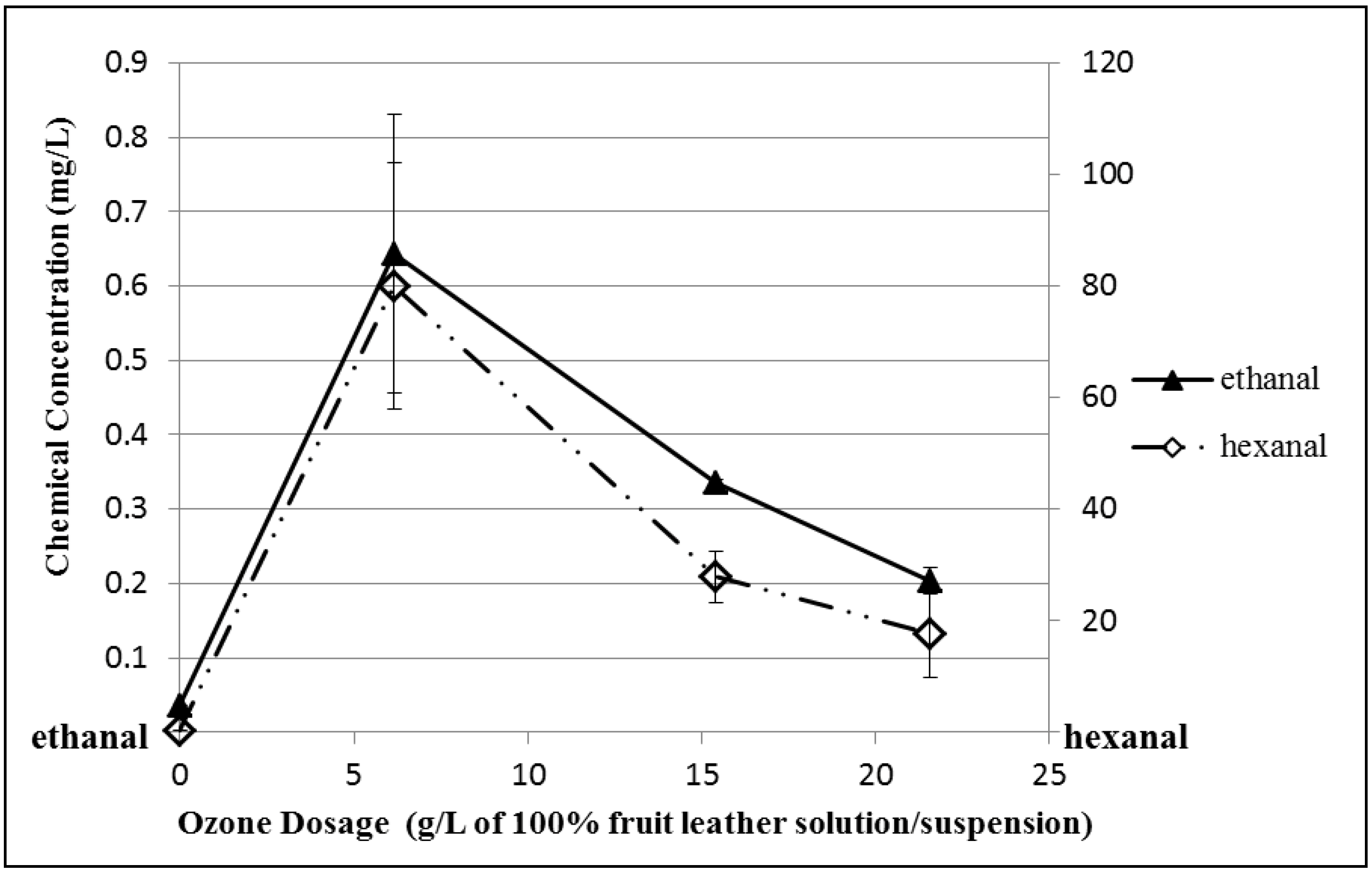
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ozsoy, H.D.; van Leeuwen, J. Removal of color from fruit candy waste by activated carbon adsorption. J. Food Eng. 2010, 101, 106–112. [Google Scholar] [CrossRef]
- Van Leeuwen, J.; Pipe-Martin, C.; Lehmann, R. The South Caboolture Water Reclamation Process. Ozone Sci. Eng. 2003, 25, 107–120. [Google Scholar] [CrossRef]
- Van Leeuwen, J.; Sridhar, A.; Esplugas, M.; Onuki, S.; Cai, L.; Harrata, A.K.; Koziel, J.A. Improving biodegradation of organic pollutants with ozonation during biological wastewater treatment. Ozone Sci. Eng. 2009, 31, 63–70. [Google Scholar] [CrossRef]
- FDA, US Food and Drug Administration. Sec. 173.368 Ozone. Part 173 D, Title 21, Secondary direct food additives permitted in food for human consumption, Specific Usage Additives. 1 April 2013. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=173.368 (accessed on 15 December 2014).
- Zhu, W.; Koziel, J.A.; Cai, L.; Brehm-Stecher, B.; Özsoy, H.D.; van Leeuwen, J. Ozonation-based decolorization of food dyes for recovery of fruit leather wastes. J. Agric. Food Chem. 2013, 61, 8198–8206. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef] [PubMed]
- FDA, US Food and Drug Administration. Sec. 182.60 Title 21,3 Substances generally recognized as safe, Synthetic flavoring substances and adjuvants. 1 April 2013. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.60 (accessed on 15 December 2014).
- Uebelacker, M.; Lachenmeier, D.W. Quantitative determination of acetaldehyde in foods using automated digestion with simulated gastric fluid followed by headspace gas chromatography. J. Autom. Method. Manag. 2011, 2011, 907317. [Google Scholar]
- FDA, US Food and Drug Administration. Sec 172.515 Title 21, 3 Synthetic flavoring substances and adjuvants. 1 April 2013. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.515 (accessed on 15 December 2014).
- Cancho, B.; Ventura, F.; Galceran, M.T. Determination of aldehydes in drinking water using pentafluorobenzylhydroxylamine derivatization and solid-phase microextraction. J. Chromatogr. A 2002, 943, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, J.; Sridhar, A.; Esplugas, M.; Onuki, S.; Cai, L.; Koziel, J.A. Ozonation within an activated sludge system for azo dye removal by partial oxidation and biodegradation. Ozone Sci. Eng. 2009, 31, 279–286. [Google Scholar] [CrossRef]
- Le Lacheur, R.M.; Sonnenberg, L.B.; Singer, P.C.; Christman, R.F.; Charles, M.J. Identification of carbonyl compounds in environmental samples. Environ. Sci. Technol. 1993, 27, 2745–2753. [Google Scholar] [CrossRef]
- Bao, M.L.; Pantani, F.; Griffini, O.; Burrini, D.; Santianni, D.; Barbieri, K. Determination of carbonyl compounds in water by derivatization-solid-phase microextraction and gas chromatographic analysis. J. Chromatogr. A 1998, 809, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sladek, N.E.; Manthey, C.L.; Maki, P.A.; Zhang, Z.; Landkamer, G.J. Xenobiotic oxidation catalyzed by aldehyde dehydrogenases. Drug Metab. Rev. 1989, 20, 697–720. [Google Scholar] [CrossRef] [PubMed]
- Chaw, Y.M.; Crane, L.E.; Lange, P.; Shapiro, R. Isolation and identification of cross-links from formaldehyde treated nucleic acid. Biochemistry 1980, 19, 5525–5531. [Google Scholar] [CrossRef] [PubMed]
- Feldman, V.J.; Kruysse, A. Effects of exposure to acrolein vapor in hamsters simultaneously treated with benzo[α]pyrene or diethylnitrosamine. J. Toxicol. Environ. Health 1978, 3, 379–394. [Google Scholar]
- Ma, T.H.; Harris, M.M. Review of the Genotoxicity of formaldehyde. Mutat. Res. 1988, 196, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Culleré, L.; Cacho, J.; Ferreira, V. Analysis for wine C5–C8 aldehydes through the determination of their O-(2,3,4,5,6-pentafluorobenzyl)oximes formed directly in the solid phase extraction cartridge. Anal. Chim. Acta 2004, 524, 201–206. [Google Scholar] [CrossRef]
- Ortiz, R.; Hagino, H.; Sekiguchi, K.; Wang, Q.; Sakamoto, K. Ambient air measurements of six bifunctional carbonyls in suburban area. Atmos. Res. 2006, 82, 709–718. [Google Scholar] [CrossRef]
- Pang, X.; Mu, Y.; Yuan, J.; He, H. Carbonyls emission from ethanol-blended gasoline and biodiesel-ethanol-diesel used in engines. Atmos. Environ. 2008, 42, 1349–1358. [Google Scholar] [CrossRef]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Chemical Industry Press: Beijing, China, 2009. [Google Scholar]
- Martos, P.A.; Pawliszyn, J. Sampling and determination of formaldehyde using solid-phase microextraction with on-fiber derivatization. Anal. Chem. 1998, 70, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, X. A simple, rapid and sensitive method for determination of aldehydes in human blood by gas chromatography/mass spectrometry and solid-phase microextraction with on-fiber derivatization. Rapid Commun. Mass Spectrom. 2004, 18, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Koziel, J.A.; Noah, J.; Pawliszyn, J. Field sampling and determination of formaldehyde in indoor air with solid-phase microextraction and on-fiber derivatization. Environ. Sci. Technol. 2001, 35, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Schmarr, H.; Sang, W.; Ganß, S.; Fischer, U.; Köpp, B.; Schulz, C.; Potouridis, T. Analysis of aldehydes via headspace SPME with on-fiber derivatization to their O-(2,3,4,5,6-pentafluorobenzyl)oxime derivatives and comprehensive 2D-GC-MS. J. Sep. Sci. 2008, 31, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Beránek, J.; Kubatova, A. Evaluation of solid-phase microextraction methods for determination of trace concentration aldehydes in aqueous solution. J. Chromatogr. A 2008, 1209, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Optimisation of a complete method for the analysis of volatiles involved in the flavour stability of beer by solid-phase microextraction in combination with gas chromatography and mass spectrometry. J. Chromatogr. A 2008, 1190, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Wardencki, W.; Sowinski, P.; Curylo, J. Evaluation of headspace solid-phase microextraction for the analysis of volatile carbonyl compounds in spirits and alcoholic beverages. J. Chromatogr. A 2003, 984, 89–96. [Google Scholar] [CrossRef] [PubMed]
- López-Vázquez, C.; Orriols, I.; Perelló, M.-C.; de Revel, G. Determination of aldehydes as pentafluorobenzyl derivatives in grape pomace distillates by HS-SPME-GC/MS. Food Chem. 2012, 130, 1127–1133. [Google Scholar] [CrossRef]
- Serrano, M.; Gallego, M.; Silva, M. Static headspace gas chromatography–mass spectrometry for the one-step derivatisation and extraction of eleven aldehydes in drinking water. J. Chromatogr. A 2013, 1307, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Koziel, J.A.; Dharmadhikari, M.; van Leeuwen, J. Rapid determination of trans-resveratrol in red wine by solid phase microextraction with on fiber derivatization and multidimensional gas chromatography-mass spectrometry. J. Chromatogr. A 2009, 1216, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Trenholm, R.A.; Rosario-Ortiz, F.L.; Snyder, S.A. Analysis of formaldehyde formation in wastewater using on-fiber derivatization–solid-phase microextraction–gas chromatography–mass spectrometry. J. Chromatogr. A 2008, 1210, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Reilly, J.O.; Pawliszyn, J. Determination of low-molecular mass aldehydes by automated headspace solid-phase microextraction with in-fibre derivatisation. J. Chromatogr. A 2005, 1071, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Chang, C.M. Analysis of aldehydes in water by solid-phase microextraction with on-fiber derivatization. J. Chromatogr. A 2003, 1015, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Munch, J.W.; Munch, D.J.; Winslow, S.D.; Wendelken, S.C.; Pepich, B.V. Determination of carbonyl compounds in drinking water by pentafluorobenzylhydroxylamine derivatization and capillary gas chromatography with electron capture detection. Method 556.1; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1998. [Google Scholar]
- Buttery, R.G.; Ling, L.C.; Guadagni, D.G. Volatilities of aldehydes, ketones, and esters in dilute water solutions. J. Agric. Food Chem. 1969, 17, 385–389. [Google Scholar] [CrossRef]
- SRC, Syracuse Res Corp; syracuse, NY, USA. Furfural, The Physical Properties Database (PHYSPROP). Available online: http://esc.syrres.com/fatepointer/webprop.asp?CAS=98011 (accessed on 7 January 2014).
- Zhou, X.; Mopper, K. Apparent partition coefficients of 15 carbonyl compounds between air and seawater and between air and freshwater; Implications for air-sea exchange. Environ. Sci. Technol. 1990, 24, 1864–1869. [Google Scholar] [CrossRef]
- PubChem, National Institutes of Health. 2-Furaldehyde. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/7362 (accessed on 15 December 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Koziel, J.A.; Cai, L.; Özsoy, H.D.; Van Leeuwen, J. Quantification of Carbonyl Compounds Generated from Ozone-Based Food Colorants Decomposition Using On-Fiber Derivatization-SPME-GC-MS. Chromatography 2015, 2, 1-18. https://doi.org/10.3390/chromatography2010001
Zhu W, Koziel JA, Cai L, Özsoy HD, Van Leeuwen J. Quantification of Carbonyl Compounds Generated from Ozone-Based Food Colorants Decomposition Using On-Fiber Derivatization-SPME-GC-MS. Chromatography. 2015; 2(1):1-18. https://doi.org/10.3390/chromatography2010001
Chicago/Turabian StyleZhu, Wenda, Jacek A. Koziel, Lingshuang Cai, H. Duygu Özsoy, and J. (Hans) Van Leeuwen. 2015. "Quantification of Carbonyl Compounds Generated from Ozone-Based Food Colorants Decomposition Using On-Fiber Derivatization-SPME-GC-MS" Chromatography 2, no. 1: 1-18. https://doi.org/10.3390/chromatography2010001
APA StyleZhu, W., Koziel, J. A., Cai, L., Özsoy, H. D., & Van Leeuwen, J. (2015). Quantification of Carbonyl Compounds Generated from Ozone-Based Food Colorants Decomposition Using On-Fiber Derivatization-SPME-GC-MS. Chromatography, 2(1), 1-18. https://doi.org/10.3390/chromatography2010001







