New Nanomaterials and Luminescent Optical Sensors for Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Probes for H2O2 Based on Nanomaterials
| Material and Type of Nanostructure | Detected Signal | Analytical Range (LOD), μM | Incubation Time and Conditions | Application to Real Sample | Ref. |
|---|---|---|---|---|---|
| Gold Nanoparticles | |||||
| Au nanodots | Luminescence quenching of Au nanodots with 11-mercapto-undecanoic acid | 0.1–1 × 103 (0.03) | 10 min in 10 mM sodium phosphate buffer (PB) of pH 5.0 at 65 °C | Glucose in serum sample | [12] |
| Dye-doped silica nanoparticles with Au nanoparticles on surface | Luminescence quenching of fluorescein isothiocyanate | 0.1–15 | 10 min in 10 mM PB of pH 7.0 at 37 °C | Detection of H2O2, hydroquinone, glucose, acetylthiocholine and paraoxon | [13] |
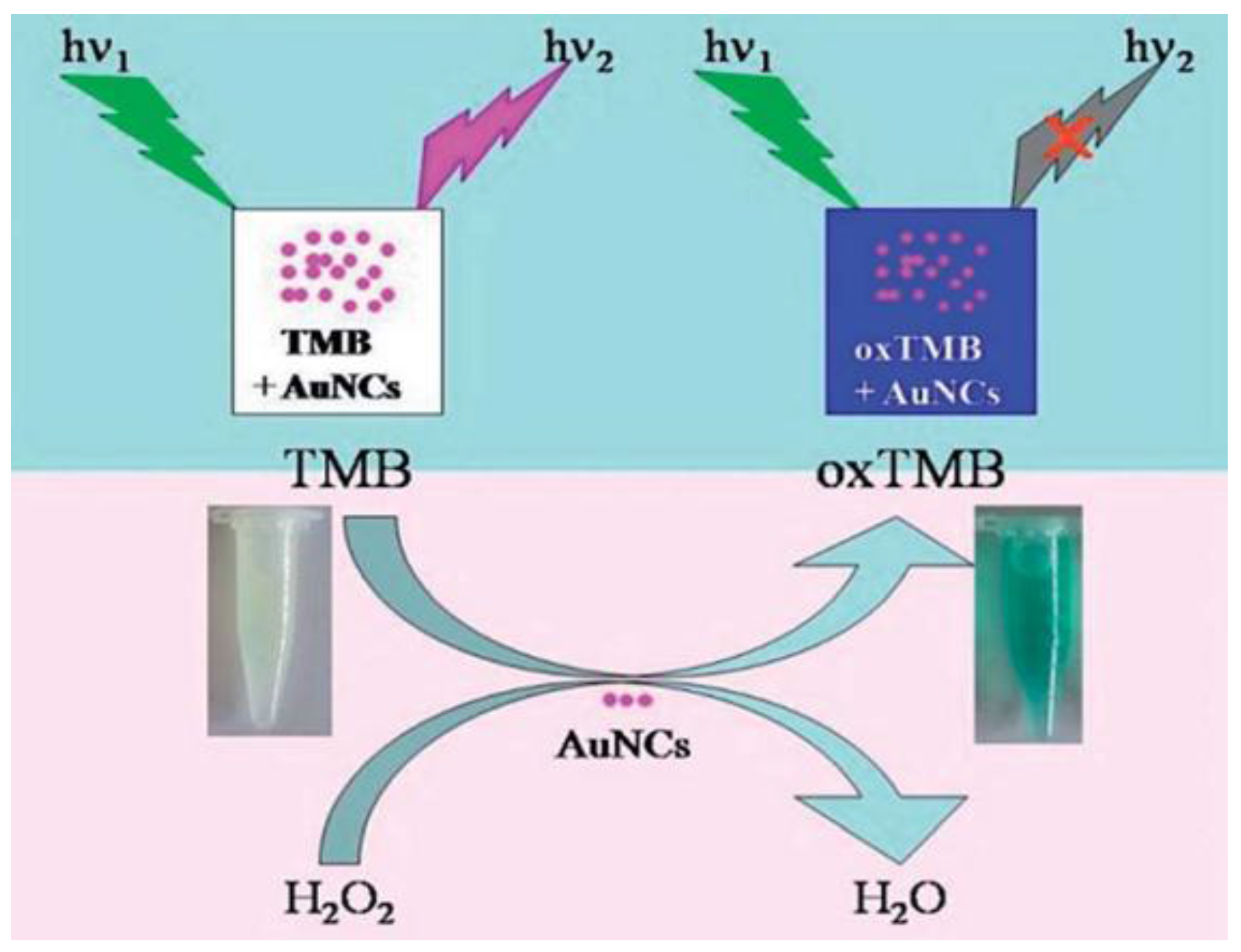
| Au nanoclusters stabilized by glutathione with peroxidase-like activity | Colorimetric and visual assay based on detection of oxidized 3,3′,5,5′-tetramethyl-benzidine (TMB) | 1–10 (0.032) | 15 min in 0.1 M PB of pH 6.0 at 30 °C | Glucose in human serum | [14] |
| Luminescence quenching of Au nanoclusters in presence of oxidized TMB | 2 × 10−3–6×10−4 (4.9 × 10−7) | 15 min in 0.1 M PB of pH 6.0 at 30 °C | |||
| Au nanoclusters bioconjugated with Horseradish peroxidase (HRP) | Luminescence quenching of Au nanoclusters | 0.1–100 (0.03) | 10 min in 50 mM glycine buffer of pH 9.0 at 25 °C | [15] | |
| Polymer-Nanoparticles with Embedded Enzymes | |||||
| HRP co-entrapped with Texas Red-dextran inside porous polyacrylamide nanoparticles | Fluorescence quenching of Texas Red due to oxidation | 1–25 | 5 min in 0.01 M phosphate-buffered saline (PBS) of pH 7.4 | Cell culture medium containing 10% blood serum | [16] |
| Aggregation-Induced Emission Enhancement | |||||
| Fluorescent dye entrapped in CTAB micelles | Aggregation induced ratiometric (510/405 nm) fluorescence switched on by excited-state intramolecular proton transfer | up to 1 × 103 | 11 min in 0.3 mM CTAB solution (20 mM HEPES buffer of pH 7.4 at 25 °C) | [17] | |
| Quantum Dots | |||||
| Nanocomposites with Fe3O4 core and CdTe shell | Quenching of luminescence of quantum dots (QD) due to etching of surface | 1 × 102–1 × 103 (35) | 15 min in water | Human urine | [18] |
| CdTe@ZnS QDs conjugated to metal tetraamino-phthalocyanines (Metal: Al, Ni, Zn) | Increasing fluorescence of QDs | (9.8 × 10−3 4.4 × 10−3 2.2 × 10−3) | 15 min in 50 mM PBS of pH 7.4 at RT | [19] | |
| Lanthanide-Based Nanoparticles | |||||
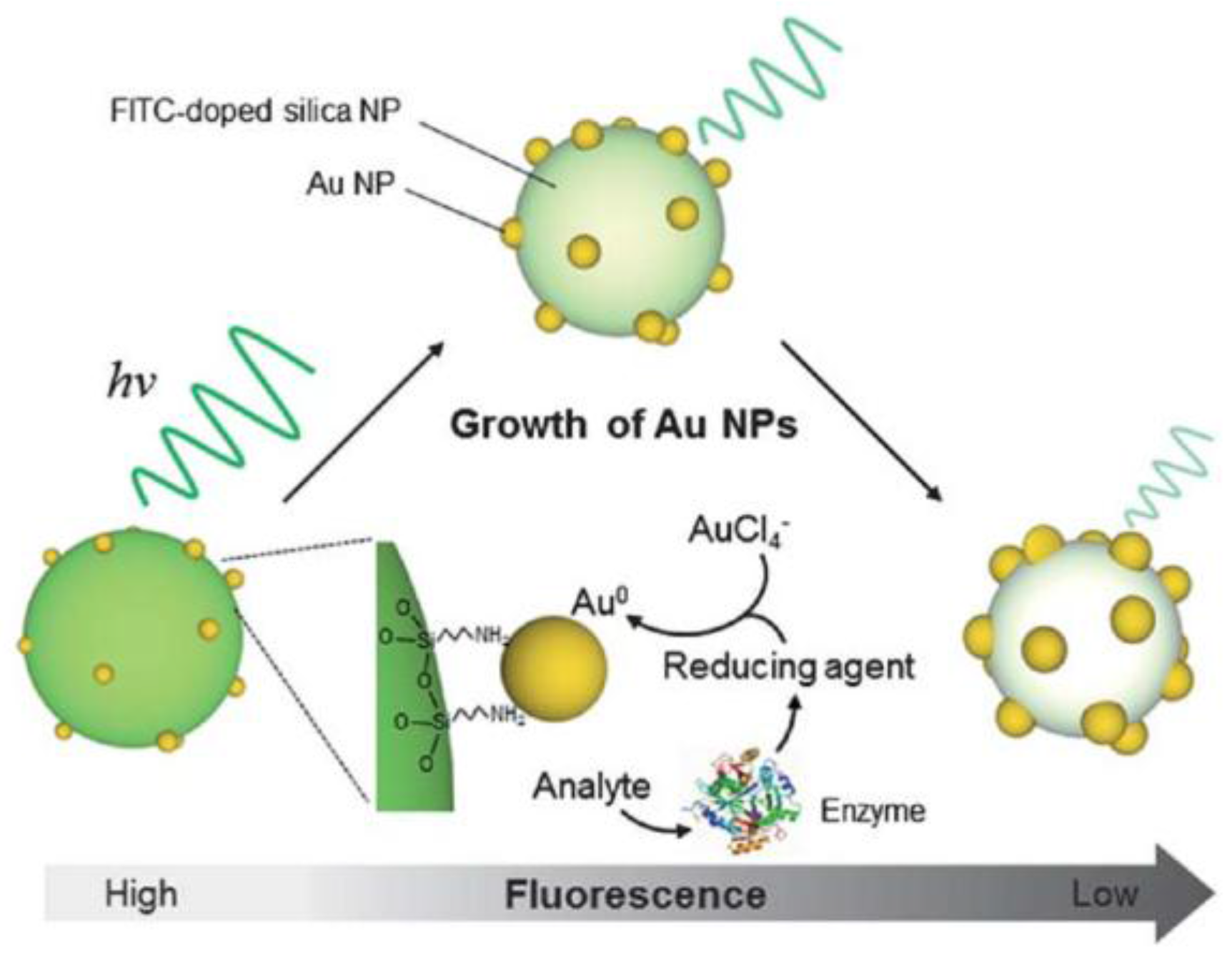
| Poly(methyl methacrylate)-Eu3+ nanospheres | Luminescence quenching with biocatalytic growth of Au nanoparticles (AuNPs) | 4.0–16 (2.0) | 5 h growth of AuNPs in 0.01 M PBS of pH 7.05 in ice–water, luminescence after 20 min at RT | [20] | |
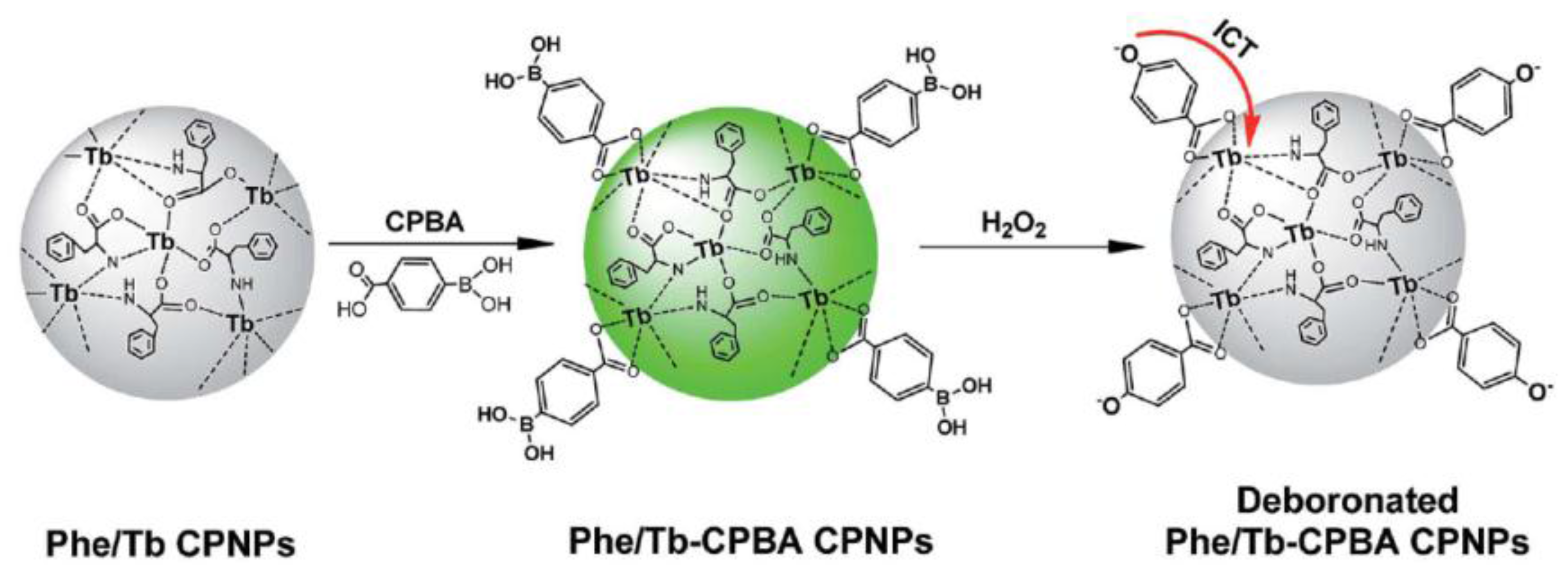
| Coordination polymer nanoparticles (phenylalanine/Tb3+) coordinated with carboxyphenyl-boronic acids | Quenching of fluorescence of nanoparticles due to intramolecular charge transfer from 4-oxo anions to emissive state of Tb3+ | 6–1 × 103 (2) | 20 min in HEPES buffer of pH 7.0 at RT | Urine samples | [21] |
| Upconversion photoluminescence nanoparticles NaYF4:Yb3+/Er3+ | Quenching of luminescence of nanoparticles in presence of oxidized TMB | 0.1–4.0 (0.045) | 10 min in 0.02 M acetate buffer of pH 5.0 at 25 °C | Glucose in human serum | [22] |
| Carbon Based Nanomaterials | |||||
| Carbon nanodots | Fluorescence quenching of nanodots in the presence of H2O2/Fe2+ | 0.025–50 (0.01) | 10 min in HCl of pH 3.0 | [23] | |
| Graphene quantum dots (GQDs) coupled with 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid (ABTS) | Absorbance change of ABTS | 102–104 (20) | 2 min in 10 mM Tris–HCl of pH 5.0 at 37 °C | [24] | |
| GQDs noncovalently labeled with hemin | Quenching of luminescence of GQDs | 1–100 (0.1) | 10 min in 20 mM PBS of pH 7.0 at RT | Glucose in human serum | [25] |
| Carboxyl-functionalized multiwalled carbon nanotubes | Fluorescence of tetraguaiacol formed from guaiacol oxidation in presence HRP | (1.2 μM × s−1) | 100 s | [26] | |
2.1. Gold Nanoparticles
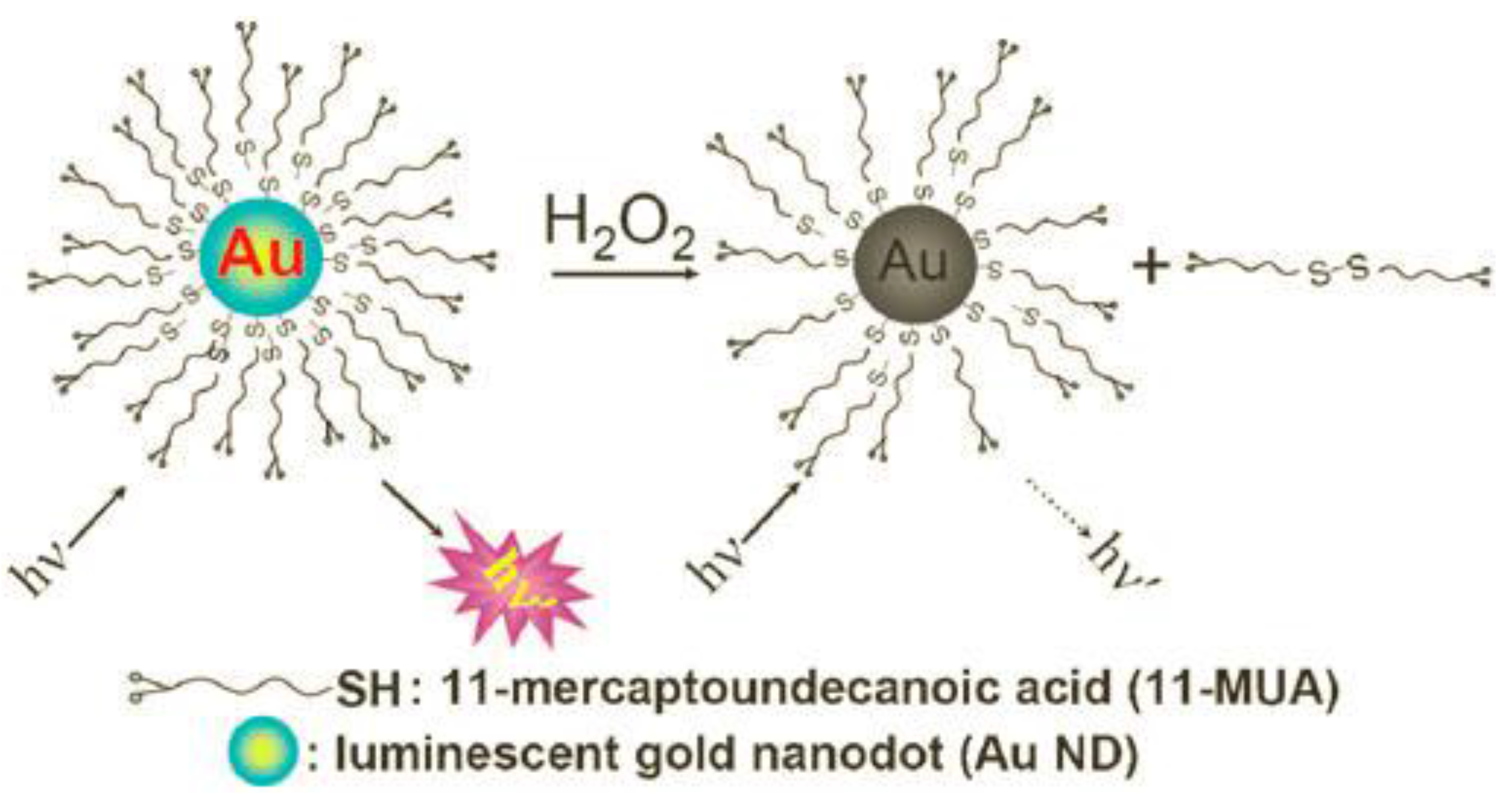
2.2. Polymer-Nanoparticles with Embedded Enzymes
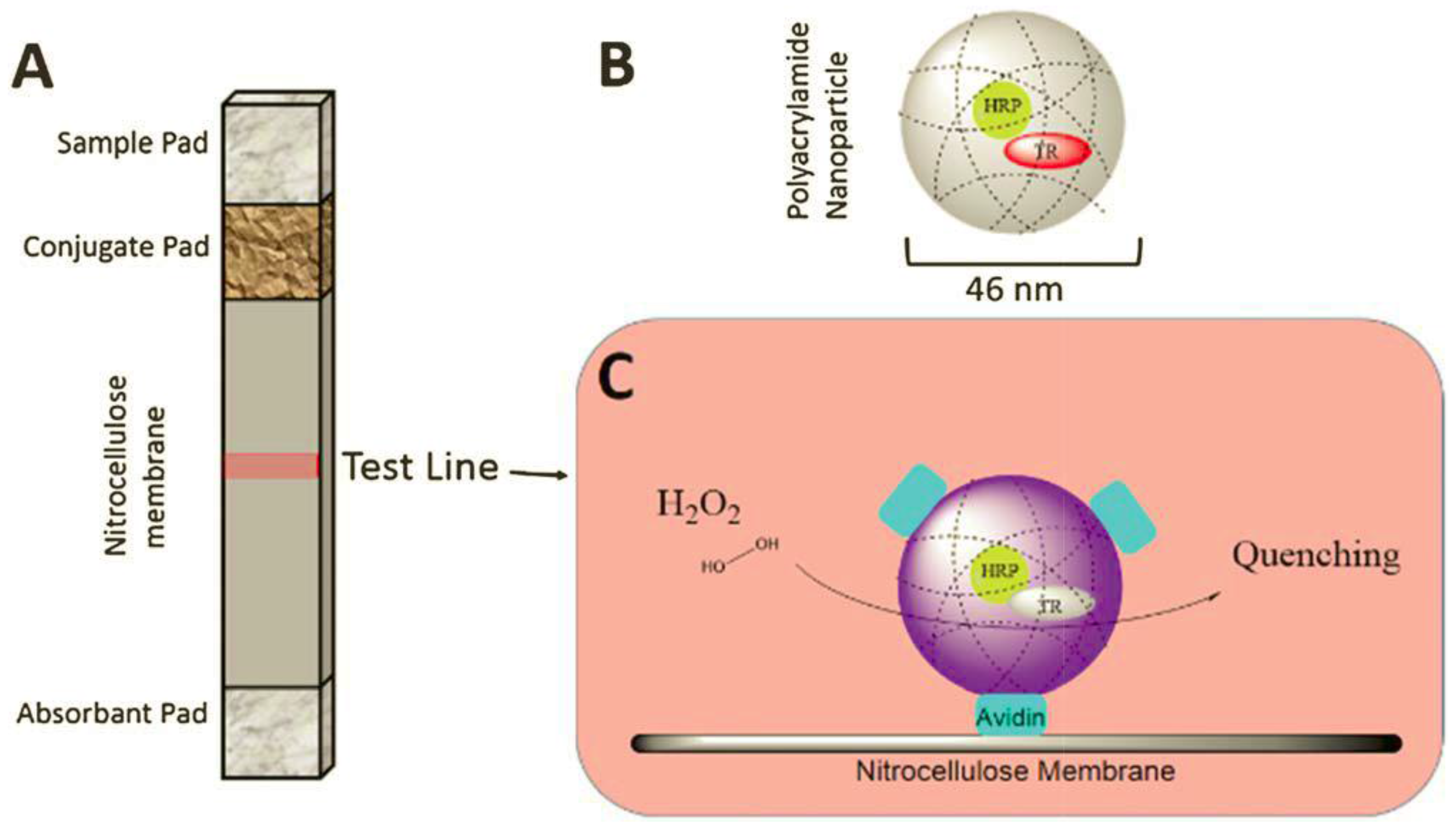
2.3. Aggregation-Induced Emission Enhancement

2.4. Quantum Dots
2.5. Lanthanide-Based Nanoparticles
2.6. Carbon Based Nanomaterials

3. Sensors, Sensing Membranes and Films for H2O2 Detection
| Composition of Film | Detected Signal | Analytical Range (LOD), mM | Response Time | Conditions and Electrolyte | Ref. |
|---|---|---|---|---|---|
| Eu3+-tetracycline complex incorporated into a polyacrylonitrile- polyacrylamide co-polymer | Luminescence increase | 0.45–10 (0.45) | 10 min | MOPS buffer of pH 6.9 at RT | [34] |
| [Ru(dpp)32+] and MnO2 as catalyst | Quenching of luminescence Ru complex due to O2 via phase angle measurement | 60–300 | 1 min | aqueous solutions at 25 °C | [36] |
| [Ru(bpy)32+(Ph4B2)2] | Luminescence quenching of the dye ion-pair due to O2 | 10–1 × 103 (1) | 5.2 min | deionized water | [37] |
| Immobilization of catalase conjugated to O2-sensitive Ru-complex in polyacrylamide | Quenching of luminescence Ru complex due to O2 formation | 0.5–14 (0.001) | 0.1 M carbonate buffer of pH 9 | [38] | |
| Disks of TiO2/SiO2 NP powder in flow-through cell | Phosphorescence quenching due to superoxide coordination to Ti | 7 × 10−4–70 1.6 × 10−4 | few seconds | water | [39] |
| HP Green incorporated into a polyurethane polymer | Photoinduced electron transfer of enzymatically oxidized HP Green | 0.03–0.3 (8 × 10−3) | 10 min | 10 mM PBS of pH 7.4 at RT | [40] |

4. Assessment of Nanomaterials for Potential Use in Optical Sensors
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 1992, 258, 1898–1902. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The Free Radical Theory of Aging Matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [PubMed]
- Giorgio, M.; Trinel, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. H2O2, a Necessary Evil for Cell Signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cai, S.; Ren, Q.Q.; Wen, W.; Zhao, Y. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Borisov, S.M.; Wolfbeis, O.S. Optical Biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Schäferling, M.; Grögel, D.B.M.; Schreml, S. Luminescent probes for detection and imaging of hydrogen peroxide. Microchim. Acta 2011, 174, 1–18. [Google Scholar] [CrossRef]
- Shiang, Y.-C.; Huang, C.-C.; Chang, H.-T. Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose. Chem. Commun. 2009, 23, 3437–3439. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Kim, J.H.; Lee, J.S.; Ahn, J.; Kim, M.-G.; Park, C.B. Multi-layered stacks of fluorescent dye-doped silica nanoparticles decorated by gold nanoparticles for solid-phase optical biosensing. J. Mater. Chem. 2011, 21, 17623–17626. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, S.; Huang, H.; Zhang, L.; Wang, L.; Liu, F.; Chen, J.; Zenga, Y.; Chu, P.K. Colorimetric and ultra-sensitive fluorescence resonance energy transfer determination of H2O2 and glucose by multi-functional Au nanoclusters. Analyst 2014, 139, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Dong, Y.; Feng, L.; Wang, S.; Zhang, S.; Zhang, X. Horseradish Peroxidase Functionalized Fluorescent Gold Nanoclusters for Hydrogen Peroxide Sensing. Anal. Chem. 2011, 83, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Özalp, V.C.; Zeydanlı, Ü.S.; Lunding, A.; Kavruk, M.; Öz, M.T.; Eyidögan, F.; Olsend, L.F.; Öktem, H.A. Nanoparticle embedded enzymes for improved lateral flow sensors. Analyst 2013, 138, 4255–4259. [Google Scholar]
- Li, G.; Zhu, D.; Liu, Q.; Xue, L.; Jiang, H. Rapid Detection of Hydrogen Peroxide Based on Aggregation Induced Ratiometric Fluorescence Change. Org. Lett. 2013, 15, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Su, R.; Gao, Z.; Qi, W.; Huang, R.; Wang, L.; Hea, Z. Magnetic–fluorescent nanocomposites as reusable fluorescence probes for sensitive detection of hydrogen peroxide and glucose. Anal. Methods 2014, 6, 6352–6357. [Google Scholar] [CrossRef]
- Adegoke, O.; Khene, S.; Nyokong, T. Fluorescence “Switch on” of Conjugates of CdTe@ZnS Quantum Dots with Al, Ni and Zn Tetraamino-Phthalocyanines by Hydrogen Peroxide: Characterization and Applications as Luminescent Nanosensors. J. Fluoresc. 2013, 23, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, H.; Shen, J. Preparation of Stable Luminescent Poly(methyl methacrylate)–Europium Complex Nanospheres and Application in the Detection of Hydrogen Peroxide with the Biocatalytic Growth of Gold Nanoparticles. J. Appl. Polym. Sci. 2013, 128, 845–850. [Google Scholar] [CrossRef]
- Tan, H.; Ma, C.; Li, Q.; Wang, L.; Xu, F.; Chen, S.; Song, Y. Functionalized lanthanide coordination polymer nanoparticles for selective sensing of hydrogen peroxide in biological fluids. Analyst 2014, 139, 5516–5522. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, L.; Li, A.; Tang, J.; Wang, S.; Xu, S.; Wang, L. Simultaneous Detection of Hydrogen Peroxide and Glucose in Human Serum with Upconversion Luminescence. Biosens. Bioelectron. 2015, 68, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Qiang, L.; Ren, J.; Ren, X.; Tanga, F.; Meng, X. Fluorescence turn-off detection of hydrogen peroxide and glucose directly using carbon nanodots as probes. Anal. Methods 2014, 6, 1922–1927. [Google Scholar] [CrossRef]
- Wu, X.; Tian, F.; Wang, W.; Chen, J.; Wub, M.; Zhao, J.X. Fabrication of highly fluorescent graphene quantum dots using l-glutamic acid for in vitro/in vivo imaging and sensing. J. Mater. Chem. C 2013, 1, 4676–4684. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; Sun, J.; Jiao, S.; Chen, H.; Gao, F.; Wang, L. Fluorescent blood glucose monitor by hemin-functionalized graphene quantum dots based sensing system. Anal. Chim. Acta 2014, 810, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Magyar, M.; Hajdu, K.; Szabó, T.; Endrödi, B.; Hernádi, K.; Horváth, E.; Magrez, A.; Forró, L.; Visy, C.; Nagy, L. Sensing hydrogen peroxide by carbon nanotube/horseradish peroxidase bio-nanocomposite. Phys. Status Solidi B 2013, 250, 2559–2563. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Díeza, I.; Ras, R.H.A. Fluorescent silver nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum dots in bioanalysis: A review of applications across various platforms for fluorescence spectroscopy and imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [PubMed]
- Vuojola, J.; Soukka, T. Luminescent lanthanide reporters: New concepts for use in bioanalytical applications. Methods Appl. Fluoresc. 2014, 2, 012001. [Google Scholar] [CrossRef]
- Wolfbeis, O.S.; Dürkop, A.; Wu, M.; Lin, Z. Europium Ion-Based Luminescent Sensing Probe for Hydrogen Peroxide. Angew. Chem. Int. Ed. 2002, 41, 4495–4498. [Google Scholar] [CrossRef]
- Deng, X.; Tang, H.; Jiang, J. Recent progress in graphene-material-based optical sensors. Anal. Bioanal. Chem. 2014, 406, 6903–6916. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S.; Schaferling, M.; Dürkop, A. Reversible Optical Sensor Membrane for Hydrogen Peroxide Using an Immobilized Fluorescent Probe, and Its Application to a Glucose Biosensor. Microchim. Acta 2003, 3, 221–227. [Google Scholar] [CrossRef]
- Posch, H.E.; Wolfbeis, O.S. Optical sensor for hydrogen peroxide. Mikrochim. Acta 1989, 97, 41–50. [Google Scholar] [CrossRef]
- Voraberger, H.S.; Trettnak, W.; Ribitsch, V. Optochemical hydrogen peroxide sensor based on oxygen detection. Sens. Actuators B Chem. 2003, 90, 324–331. [Google Scholar] [CrossRef]
- Mills, A.; Tommons, C.; Bailey, R.; Tedford, M.C.; Crilly, P.J. Reversible, fluorescence-based optical sensor for hydrogen peroxide. Analyst 2007, 132, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; de Marcos, S.; Galban, J. Fluorometric enzymatic autoindicating biosensor for H2O2 determination based on modified catalase. Biosens. Bioelectron. 2013, 41, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Chen, Y.; Yuan, H.; Gao, S.; Xiao, D. H2O2 Sensor Based on the Room-Temperature Phosphorescence of Nano TiO2/SiO2 Composite. Anal. Chem. 2007, 79, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, N.A.; Meier, R.J.; Schreml, S.; Duerkop, A. Reusable optical sensing microplate for hydrogen peroxide using a fluorescent photoinduced electron transfer probe (HP Green). Sens. Actuators B Chem. 2014, 193, 799–805. [Google Scholar] [CrossRef]
- Groegel, D.B.M.; Link, M.; Duerkop, A.; Wolfbeis, O.S. A New Fluorescent PET Probe for Hydrogen Peroxide and its Use in Enzymatic Assays for l-Lactate and d-Glucose. ChemBioChem 2011, 12, 2779–2785. [Google Scholar] [CrossRef] [PubMed]
- Ast, S.; Schwarze, T.; Müller, H.; Sukhanov, A.; Michaelis, S.; Wegener, J.; Wolfbeis, O.S.; Körzdörfer, T.; Dürkop, A.; Holdt, H.-J. A Highly K+-Selective Phenylaza-[18]crown-6-Lariat-Ether-Based Fluoroionophore and Its Application in the Sensing of K+ Ions with an Optical Sensor Film and in Cells. Chem. Eur. J. 2013, 19, 14911–14917. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burmistrova, N.A.; Kolontaeva, O.A.; Duerkop, A. New Nanomaterials and Luminescent Optical Sensors for Detection of Hydrogen Peroxide. Chemosensors 2015, 3, 253-273. https://doi.org/10.3390/chemosensors3040253
Burmistrova NA, Kolontaeva OA, Duerkop A. New Nanomaterials and Luminescent Optical Sensors for Detection of Hydrogen Peroxide. Chemosensors. 2015; 3(4):253-273. https://doi.org/10.3390/chemosensors3040253
Chicago/Turabian StyleBurmistrova, Natalia A., Olga A. Kolontaeva, and Axel Duerkop. 2015. "New Nanomaterials and Luminescent Optical Sensors for Detection of Hydrogen Peroxide" Chemosensors 3, no. 4: 253-273. https://doi.org/10.3390/chemosensors3040253





