Quantitative Analysis of the Human Milk Whey Proteome Reveals Developing Milk and Mammary-Gland Functions across the First Year of Lactation
Abstract
:1. Introduction
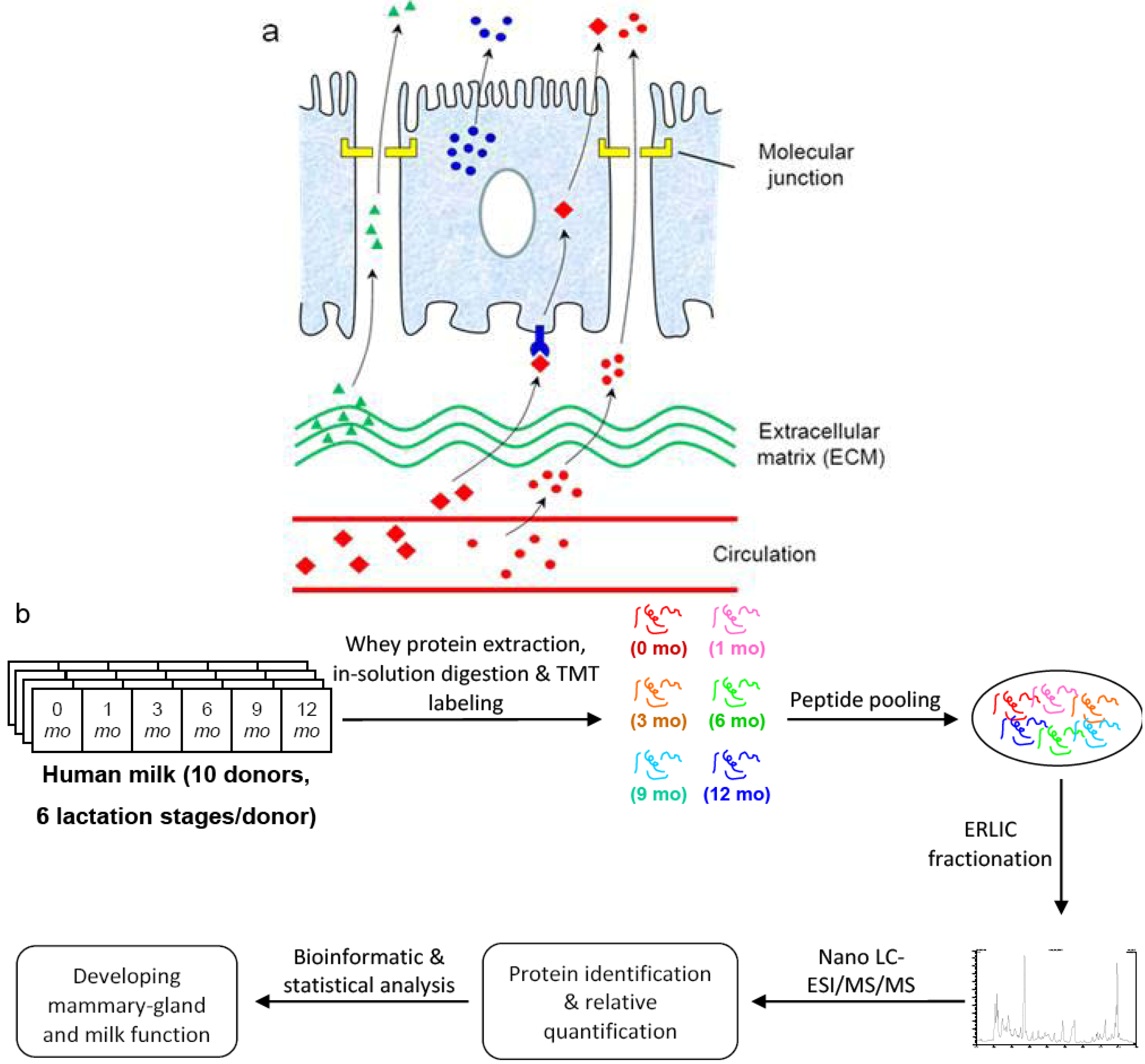
2. Experimental
2.1. Chemicals
2.2. Milk Samples
2.3. In-Solution Tryptic Digestion, Isobaric TMT Labeling and ERLIC Fractionation
2.4. Protein Digest Analysis by Nanocapillary Chromatography and Mass Spectrometry
2.5. Protein Identification and Quantification
2.6. Data Analysis
2.7. Bioinformatics Analysis
2.8. Immunoblotting Analysis
3. Results
3.1. Quantitative Human Milk Proteome during the First Year of Lactation
3.2. Transforming Human Milk Proteome throughout the First Year of Lactation
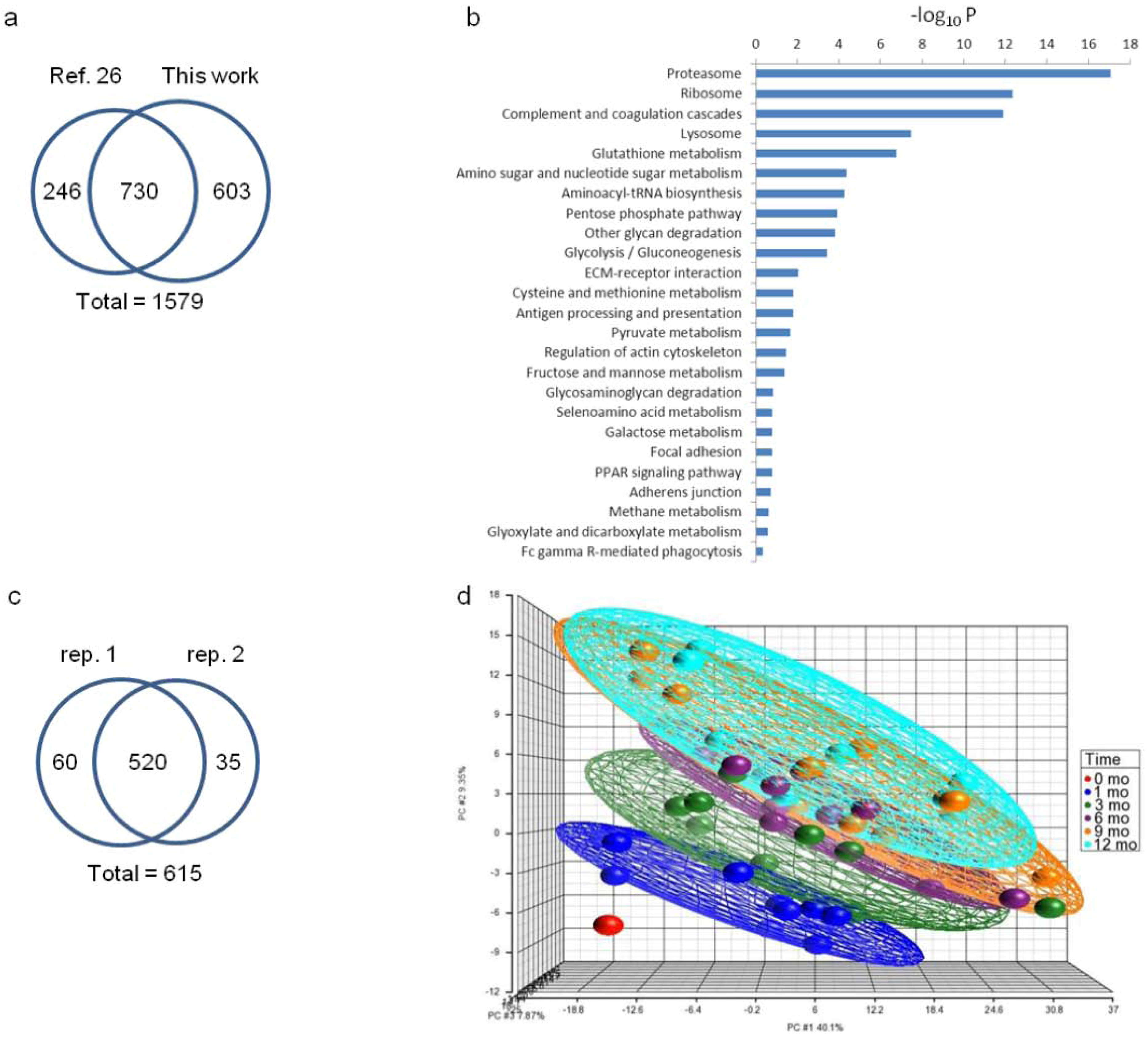
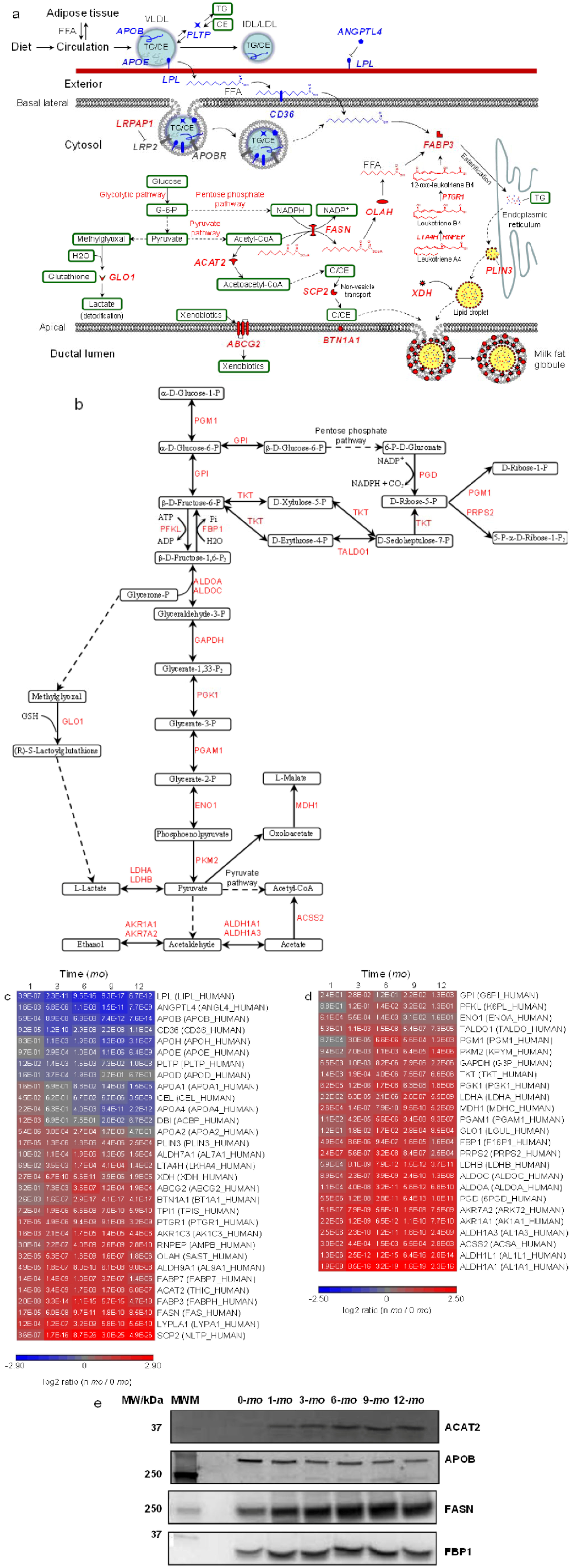
3.3. Abundance Changes of Proteins Involved in Fatty-Acid and Carbohydrate Metabolism
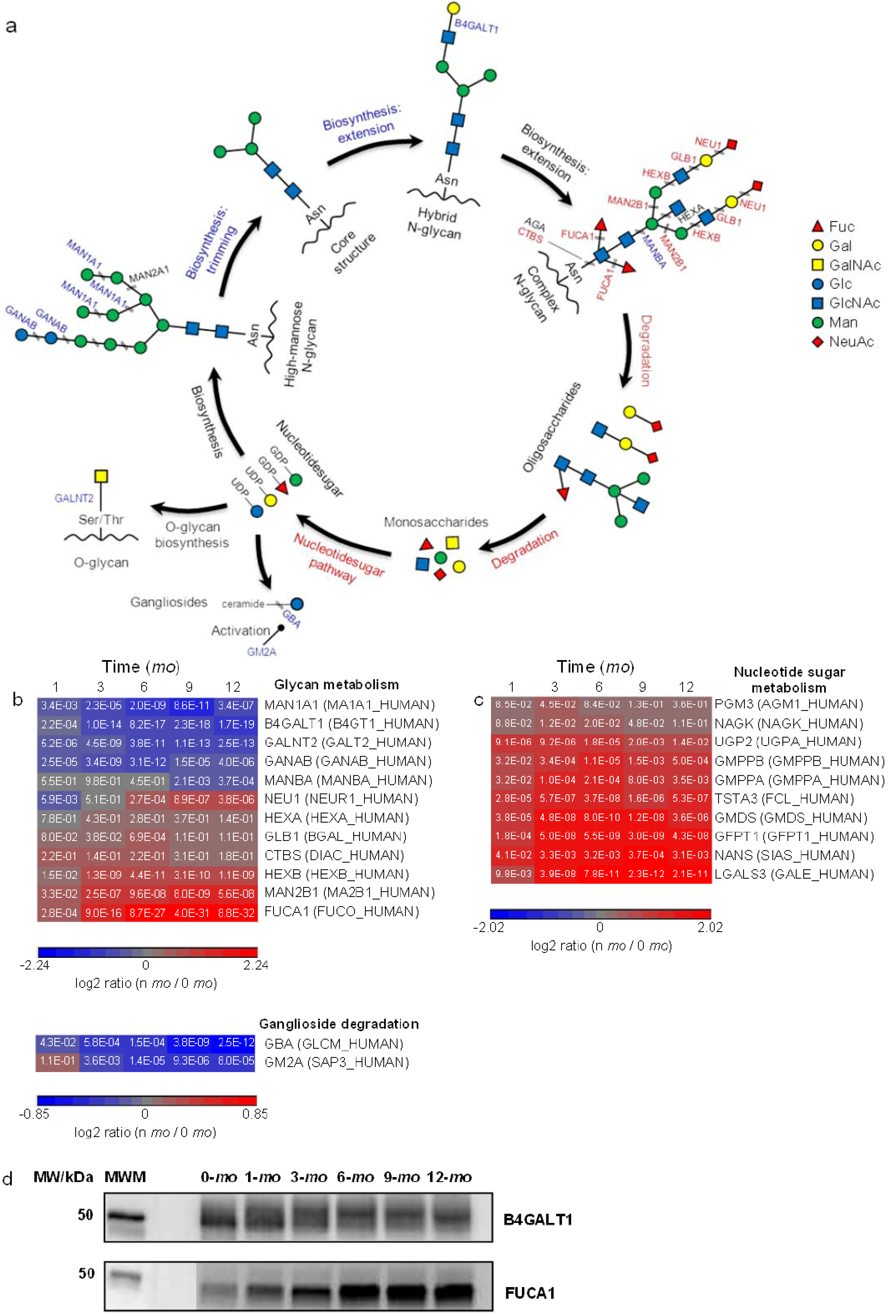
3.4. Abundance Changes of Proteins Involved in Glycoprotein Metabolism
3.5. Abundance Changes of Proteins Involved in Detoxification Processes
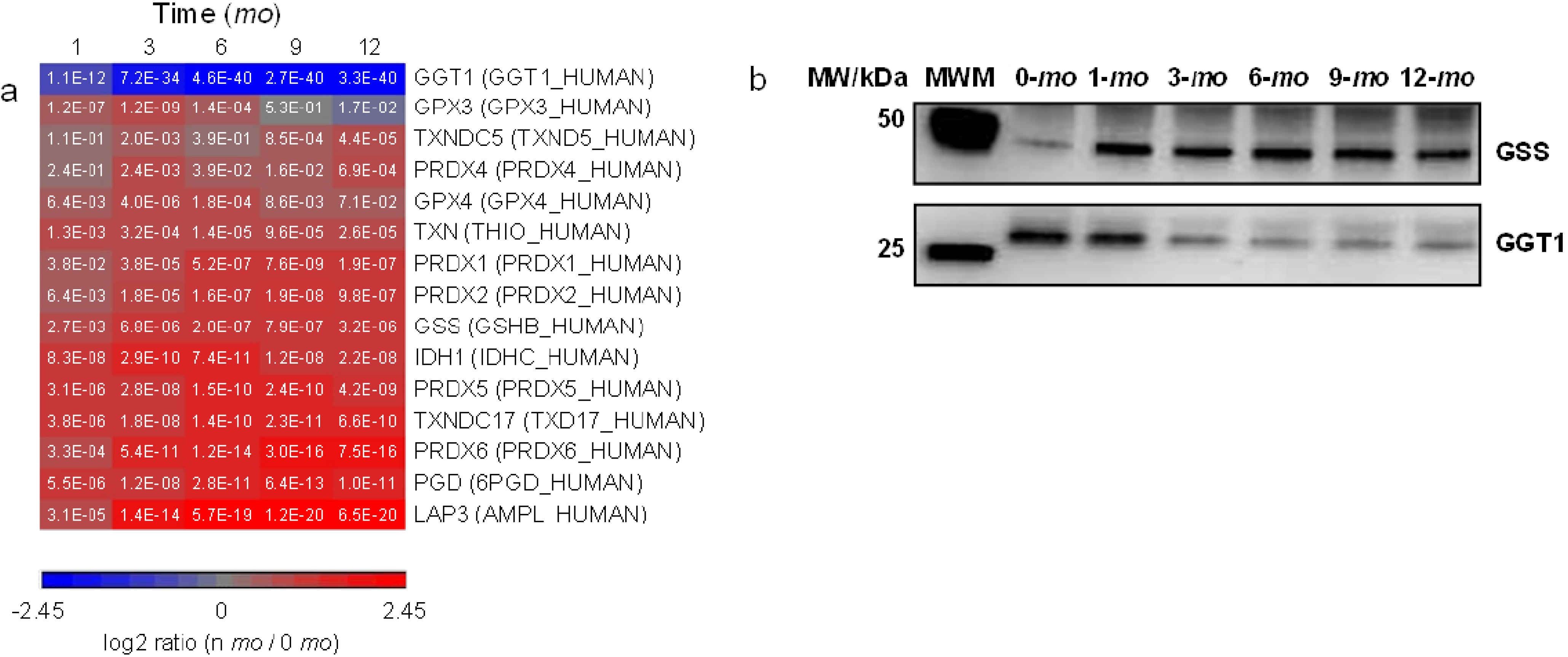
3.6. Milk Immunoglobulins
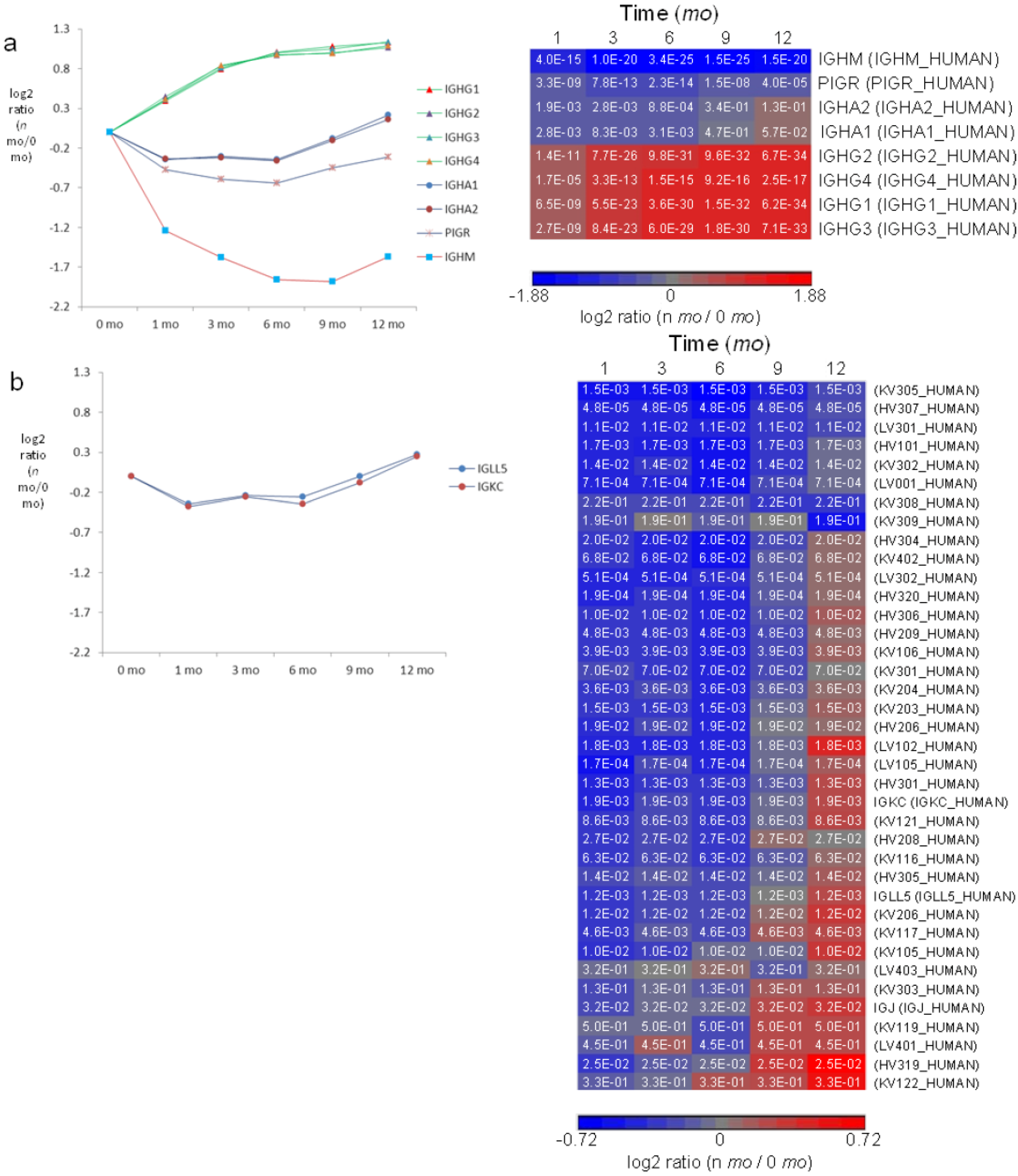
3.7. Both Classical and Alternative Pathways of the Complement Cascade are Present in Milk with a Higher Extent in Early Lactation
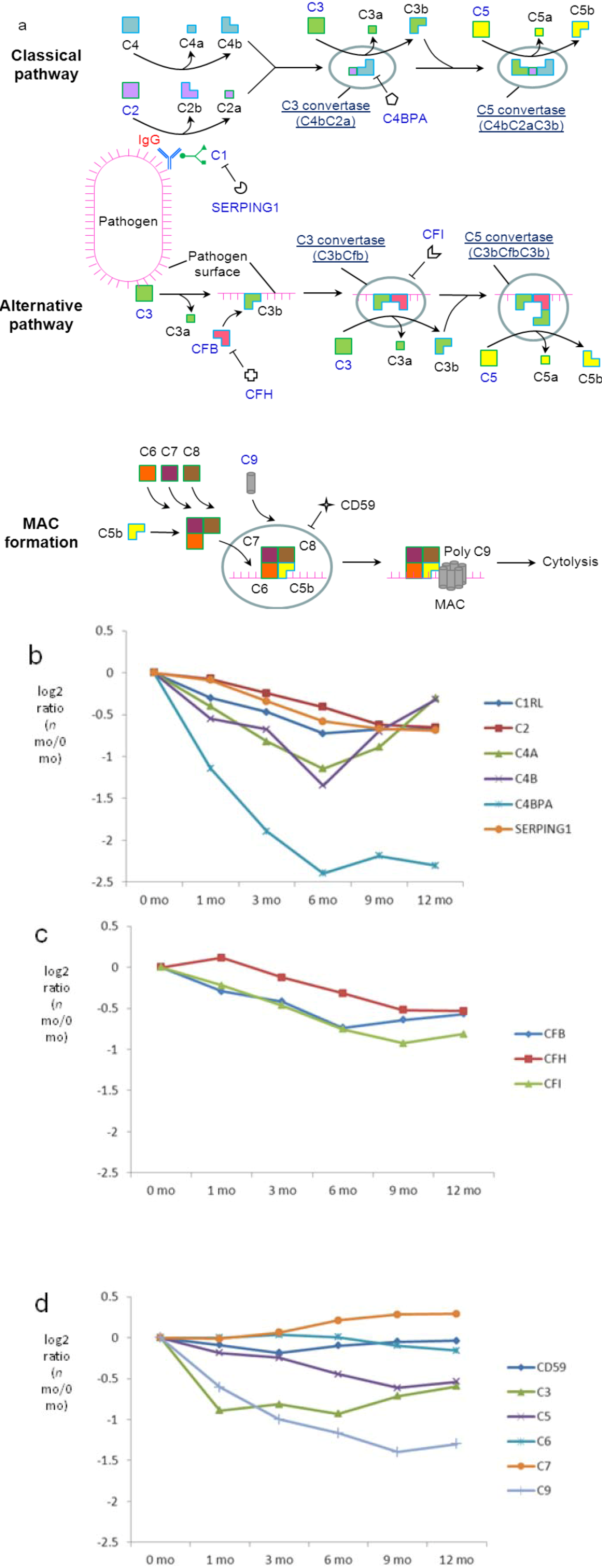
4. Discussion
5. Summary and Future Perspectives
Supplementary Materials
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Carpenter, C.J. Proteomics in milk and milk processing. In Proteomics in Foods; Toldrá, F., Nollet, L.M.L., Eds.; Springer: New York, NY, USA, 2013; pp. 223–245. [Google Scholar]
- Bauman, D.E.; Mather, I.H.; Wall, R.J.; Lock, A.L. Major advances associated with the biosynthesis of milk. J. Dairy Sci. 2006, 89, 1235–1243. [Google Scholar] [CrossRef]
- Ollier, S.; Robert-Granie, C.; Bernard, L.; Chilliard, Y.; Leroux, C. Mammary transcriptome analysis of food-deprived lactating goats highlights genes involved in milk secretion and programmed cell death. J. Nutr. 2007, 137, 560–567. [Google Scholar]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics 2008, 9, e366. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Ruminant metabolic systems biology: Reconstruction and integration of Transcriptome dynamics underlying functional responses of tissues to nutrition and physiological state. Gene Regul. Syst. Biol. 2012, 6, 109–125. [Google Scholar]
- Elsik, C.G.; Tellam, R.L.; Worley, K.C.; Gibbs, R.A.; Muzny, D.M.; Weinstock, G.M.; Adelson, D.L.; Eichler, E.E.; Elnitski, L.; Guigo, R.; et al. The genome sequence of taurine cattle: A window to ruminant biology and evolution. Science 2009, 324, 522–528. [Google Scholar] [CrossRef]
- Heid, H.W.; Keenan, T.W. Intracellular origin and secretion of milk fat globules. Eur. J. Cell Biol. 2005, 84, 245–258. [Google Scholar] [CrossRef]
- Heird, W.C.; Schwarz, S.M.; Hansen, I.H. Colostrum-induced enteric mucosal growth in beagle puppies. Pediatr. Res. 1984, 18, 512–515. [Google Scholar] [CrossRef]
- Goldman, A.S. The immune system of human milk: Antimicrobial, antiinflammatory and immunomodulating properties. Pediatr. Infect. Dis. J. 1993, 12, 664–671. [Google Scholar] [CrossRef]
- Hamosh, M. Bioactive factors in human milk. Pediatr. Clin. North Am. 2001, 48, 69–86. [Google Scholar] [CrossRef]
- Karhumaa, P.; Leinonen, J.; Parkkila, S.; Kaunisto, K.; Tapanainen, J.; Rajaniemi, H. The identification of secreted carbonic anhydrase VI as a constitutive glycoprotein of human and rat milk. Proc. Natl. Acad. Sci. USA 2001, 98, 11604–11608. [Google Scholar] [CrossRef]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Bjorck, I.M. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. [Google Scholar]
- Schack-Nielsen, L.; Michaelsen, K.F. Advances in our understanding of the biology of human milk and its effects on the offspring. J. Nutr. 2007, 137, 503S–510S. [Google Scholar]
- Pal, S.; Ellis, V. Acute effects of whey protein isolate on blood pressure, vascular function and inflammatory markers in overweight postmenopausal women. Br. J. Nutr. 2011, 105, 1512–1519. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 4653–4658. [Google Scholar]
- Fortunato, D.; Giuffrida, M.G.; Cavaletto, M.; Garoffo, L.P.; Dellavalle, G.; Napolitano, L.; Giunta, C.; Fabris, C.; Bertino, E.; Coscia, A.; et al. Structural proteome of human colostral fat globule membrane proteins. Proteomics 2003, 3, 897–905. [Google Scholar] [CrossRef]
- Cavaletto, M.; Giuffrida, M.G.; Conti, A. The proteomic approach to analysis of human milk fat globule membrane. Clin. Chim. Acta 2004, 347, 41–48. [Google Scholar] [CrossRef]
- Palmer, D.J.; Kelly, V.C.; Smit, A.M.; Kuy, S.; Knight, C.G.; Cooper, G.J. Human colostrum: Identification of minor proteins in the aqueous phase by proteomics. Proteomics 2006, 6, 2208–2216. [Google Scholar] [CrossRef]
- Mangé, A.; Bellet, V.; Tuaillon, E.; van de Perre, P.; Solassol, J. Comprehensive proteomic analysis of the human milk proteome: Contribution of protein fractionation. J. Chromatogr. B 2008, 876, 252–256. [Google Scholar] [CrossRef]
- Picariello, G.; Ferranti, P.; Mamone, G.; Roepstorff, P.; Addeo, F. Identification of N-linked glycoproteins in human milk by hydrophilic interaction liquid chromatography and mass spectrometry. Proteomics 2008, 8, 3833–3847. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Scaloni, A.; Zolla, L. Human milk proteins: An interactomics and updated functional overview. J. Proteome Res. 2010, 9, 3339–3373. [Google Scholar] [CrossRef]
- Hettinga, K.; van Valenberg, H.; de Vries, S.; Boeren, S.; van Hooijdonk, T.; van Arendonk, J.; Vervoort, J. The host defense proteome of human and bovine milk. PLoS One 2011, 6, e19433. [Google Scholar] [CrossRef]
- Liao, Y.; Alvarado, R.; Phinney, B.; Lonnerdal, B. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J. Proteome Res. 2011, 10, 3530–3541. [Google Scholar] [CrossRef]
- Liao, Y.; Alvarado, R.; Phinney, B.; Lonnerdal, B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J. Proteome Res. 2011, 10, 1746–1754. [Google Scholar] [CrossRef]
- Lu, J.; Boeren, S.; de Vries, S.C.; van Valenberg, H.J.; Vervoort, J.; Hettinga, K. Filter-aided sample preparation with dimethyl labeling to identify and quantify milk fat globule membrane proteins. J. Proteomics 2011, 75, 34–43. [Google Scholar] [CrossRef]
- Gao, X.; McMahon, R.J.; Woo, J.G.; Davidson, B.S.; Morrow, A.L.; Zhang, Q. Temporal changes in milk proteomes reveal developing milk functions. J. Proteome Res. 2012, 11, 3897–3907. [Google Scholar] [CrossRef]
- Goldman, A.S.; Garza, C.; Nichols, B.L.; Goldblum, R.M. Immunologic factors in human milk during the first year of lactation. J. Pediatr. 1982, 100, 563–567. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. B 1995, 57, 289–300. [Google Scholar]
- Szklarczyk, D.; Franceschini, A.; Kuhn, M.; Simonovic, M.; Roth, A.; Minguez, P.; Doerks, T.; Stark, M.; Muller, J.; Bork, P.; et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011, 39, D561–D568. [Google Scholar] [CrossRef]
- Alpert, A.J. Electrostatic repulsion hydrophilic interaction chromatography for isocratic separation of charged solutes and selective isolation of phosphopeptides. Anal. Chem. 2008, 80, 62–76. [Google Scholar] [CrossRef]
- Hao, P.; Qian, J.; Ren, Y.; Sze, S.K. Electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) versus strong cation exchange (SCX) for fractionation of iTRAQ-labeled peptides. J. Proteome Res. 2011, 10, 5568–5574. [Google Scholar] [CrossRef]
- Hao, P.; Guo, T.; Li, X.; Adav, S.S.; Yang, J.; Wei, M.; Sze, S.K. Novel application of electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) in shotgun proteomics: Comprehensive profiling of rat kidney proteome. J. Proteome Res. 2010, 9, 3520–3526. [Google Scholar] [CrossRef]
- De Jong, E.P.; Griffin, T.J. Online nanoscale ERLIC-MS outperforms RPLC-MS for shotgun proteomics in complex mixtures. J. Proteome Res. 2012, 11, 5059–5064. [Google Scholar] [CrossRef]
- Le, A.; Barton, L.D.; Sanders, J.T.; Zhang, Q. Exploration of bovine milk proteome in colostral and mature whey using an ion-exchange approach. J. Proteome Res. 2011, 10, 692–704. [Google Scholar] [CrossRef]
- Emken, E.A.; Adlof, R.O.; Hachey, D.L.; Garza, C.; Thomas, M.R.; Brown-Booth, L. Incorporation of deuterium-labeled fatty acids into human milk, plasma, and lipoprotein phospholipids and cholesteryl esters. J. Lipid Res. 1989, 30, 395–402. [Google Scholar]
- Olofsson, S.O.; Boström, P.; Lagerstedt, J.; Andersson, L.; Adiels, M.; Perman, J.; Rutberg, M.; Li, L.; Borén, J. The lipid droplet: A dynamic organelle, not only involved in the storage and turnover of lipids. In Cellular Lipid Metabolism; Ehnholm, C., Ed.; Springer: New York, NY, USA, 2009; pp. 1–26. [Google Scholar]
- Doege, H.; Stahl, A. Protein-mediated fatty acid uptake: Novel insights from in vivo models. Physiology (Bethesda) 2006, 21, 259–268. [Google Scholar] [CrossRef]
- Huuskonen, J.; Olkkonen, V.M.; Jauhiainen, M.; Ehnholm, C. The impact of phospholipid transfer protein (PLTP) on HDL metabolism. Atherosclerosis 2001, 155, 269–281. [Google Scholar] [CrossRef]
- Heeren, J.; Beisiegel, U. Receptor-mediated endocytosis and intracellular trafficking of lipoproteins. In Cellular Lipid Metabolism; Ehnholm, C., Ed.; Springer: New York, NY, USA, 2009; pp. 213–235. [Google Scholar]
- Kersten, S. Angiopoietin-like proteins and lipid metabolism. In Cellular Lipid Metabolism; Ehnholm, C., Ed.; Springer: New York, NY, USA, 2009; pp. 237–249. [Google Scholar]
- Hachey, D.L.; Silber, G.H.; Wong, W.W.; Garza, C. Human lactation. II: Endogenous fatty acid synthesis by the mammary gland. Pediatr. Res. 1989, 25, 63–68. [Google Scholar] [CrossRef]
- Cases, S.; Novak, S.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Welch, C.B.; Lusis, A.J.; Spencer, T.A.; Krause, B.R.; et al. ACAT-2, a second mammalian acyl-CoA: Cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 1998, 273, 26755–26764. [Google Scholar] [CrossRef]
- Wustner, D.; Herrmann, A.; Hao, M.; Maxfield, F.R. Rapid nonvesicular transport of sterol between the plasma membrane domains of polarized hepatic cells. J. Biol. Chem. 2002, 277, 30325–30336. [Google Scholar] [CrossRef]
- Prinz, W.A. Lipid trafficking sans vesicles: Where, why, how? Cell 2010, 143, 870–874. [Google Scholar] [CrossRef]
- Robenek, H.; Hofnagel, O.; Buers, I.; Lorkowski, S.; Schnoor, M.; Robenek, M.J.; Heid, H.; Troyer, D.; Severs, N.J. Butyrophilin controls milk fat globule secretion. Proc. Natl. Acad. Sci. USA 2006, 103, 10385–10390. [Google Scholar]
- Rojas, R.; Apodaca, G. Immunoglobulin transport across polarized epithelial cells. Nat. Rev. Mol. Cell Biol. 2002, 3, 944–955. [Google Scholar] [CrossRef]
- Jones, D.P. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzymol. 2002, 348, 93–112. [Google Scholar] [CrossRef]
- West, M.B.; Segu, Z.M.; Feasley, C.L.; Kang, P.; Klouckova, I.; Li, C.; Novotny, M.V.; West, C.M.; Mechref, Y.; Hanigan, M.H. Analysis of site-specific glycosylation of renal and hepatic gamma-glutamyl transpeptidase from normal human tissue. J. Biol. Chem. 2010, 285, 29511–29524. [Google Scholar] [CrossRef]
- Inoue, Y.; Kimura, A. Methylglyoxal and regulation of its metabolism in microorganisms. Adv. Microb. Physiol. 1995, 37, 177–227. [Google Scholar] [CrossRef]
- Van Herwaarden, A.E.; Wagenaar, E.; Merino, G.; Jonker, J.W.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol. Cell. Biol. 2007, 27, 1247–1253. [Google Scholar] [CrossRef]
- Bosch, T.M.; Kjellberg, L.M.; Bouwers, A.; Koeleman, B.P.; Schellens, J.H.; Beijnen, J.H.; Smits, P.H.; Meijerman, I. Detection of single nucleotide polymorphisms in the ABCG2 gene in a Dutch population. Am. J. Pharmacogenomics 2005, 5, 123–131. [Google Scholar] [CrossRef]
- Vlaming, M.L.; Lagas, J.S.; Schinkel, A.H. Physiological and pharmacological roles of ABCG2 (BCRP): Recent findings in Abcg2 knockout mice. Adv. Drug Deliv. Rev. 2009, 61, 14–25. [Google Scholar] [CrossRef]
- Field, C.J. The immunological components of human milk and their effect on immune development in infants. J. Nutr. 2005, 135, 1–4. [Google Scholar]
- Murphy, K.P. Janeway’s Immunobiology, 8th ed.; Garland Science: London, UK, 2012; pp. 48–71, 527–528. [Google Scholar]
- Hachey, D.L.; Thomas, M.R.; Emken, E.A.; Garza, C.; Brown-Booth, L.; Adlof, R.O.; Klein, P.D. Human lactation: Maternal transfer of dietary triglycerides labeled with stable isotopes. J. Lipid Res. 1987, 28, 1185–1192. [Google Scholar]
- Insull, W., Jr.; Hirsch, J.; James, T.; Ahrens, E.H., Jr. The fatty acids of human milk. II. Alterations produced by manipulation of caloric balance and exchange of dietary fats. J. Clin. Invest. 1959, 38, 443–450. [Google Scholar] [CrossRef]
- Neville, M.C.; Picciano, M.F. Regulation of milk lipid secretion and composition. Annu. Rev. Nutr. 1997, 17, 159–183. [Google Scholar] [CrossRef]
- Kay, J.K.; Weber, W.J.; Moore, C.E.; Bauman, D.E.; Hansen, L.B.; Chester-Jones, H.; Crooker, B.A.; Baumgard, L.H. Effects of week of lactation and genetic selection for milk yield on milk fatty acid composition in Holstein cows. J. Dairy Sci. 2005, 88, 3886–3893. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bernard, L.; Leroux, C.; Chilliard, Y. Role of trans fatty acids in the nutritional regulation of mammary lipogenesis in ruminants. Animal 2010, 4, 1140–1166. [Google Scholar] [CrossRef]
- Mach, N.; van Baal, J.; Kruijt, L.; Jacobs, A.; Smits, M. Dietary unsaturated fatty acids affect the mammary gland integrity and health in lactating dairy cows. BMC Proc. 2011, 5. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef]
- Carlson, S.E. N-Acetylneuraminic acid concentrations in human milk oligosaccharides and glycoproteins during lactation. Am. J. Clin. Nutr. 1985, 41, 720–726. [Google Scholar]
- Chichlowski, M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. The influence of milk oligosaccharides on microbiota of infants: Opportunities for formulas. Annu. Rev. Food Sci. Technol. 2011, 2, 331–351. [Google Scholar] [CrossRef]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef]
- Page, M.J.; Amess, B.; Townsend, R.R.; Parekh, R.; Herath, A.; Brusten, L.; Zvelebil, M.J.; Stein, R.C.; Waterfield, M.D.; Davies, S.C.; et al. Proteomic definition of normal human luminal and myoepithelial breast cells purified from reduction mammoplasties. Proc. Natl. Acad. Sci. USA 1999, 96, 12589–12594. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Mottaz, H.M.; Yu, L.R.; Anderson, D.J.; Moore, R.J.; Chen, W.N.; Auberry, K.J.; Strittmatter, E.F.; Monroe, M.E.; Thrall, B.D.; et al. Multidimensional proteome analysis of human mammary epithelial cells. J. Proteome Res. 2004, 3, 68–75. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Waters, K.M.; Kathmann, L.E.; Camp, D.G., 2nd; Wiley, H.S.; Smith, R.D.; Thrall, B.D. The mammary epithelial cell secretome and its regulation by signal transduction pathways. J. Proteome Res. 2008, 7, 558–569. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Q.; Cundiff, J.K.; Maria, S.D.; McMahon, R.J.; Woo, J.G.; Davidson, B.S.; Morrow, A.L. Quantitative Analysis of the Human Milk Whey Proteome Reveals Developing Milk and Mammary-Gland Functions across the First Year of Lactation. Proteomes 2013, 1, 128-158. https://doi.org/10.3390/proteomes1020128
Zhang Q, Cundiff JK, Maria SD, McMahon RJ, Woo JG, Davidson BS, Morrow AL. Quantitative Analysis of the Human Milk Whey Proteome Reveals Developing Milk and Mammary-Gland Functions across the First Year of Lactation. Proteomes. 2013; 1(2):128-158. https://doi.org/10.3390/proteomes1020128
Chicago/Turabian StyleZhang, Qiang, Judy K. Cundiff, Sarah D. Maria, Robert J. McMahon, Jessica G. Woo, Barbara S. Davidson, and Ardythe L. Morrow. 2013. "Quantitative Analysis of the Human Milk Whey Proteome Reveals Developing Milk and Mammary-Gland Functions across the First Year of Lactation" Proteomes 1, no. 2: 128-158. https://doi.org/10.3390/proteomes1020128



