Reversible Gelation System for Hydrazine Based on Polymer Absorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Polymers by Radical Polymerization (Typical Procedure)
2.2.2. Synthesis of Cross-Linked PVA (CPVA)
2.2.3. Gelation of Hydrazine with Absorbents
2.2.4. Releasing of Hydrazine from Hydrazine gels by N2 Gas Flow (Typical Procedure)
2.2.5. Releasing of Hydrazine from Hydrazine Gels by Mechanical Compression
3. Results and Discussion
3.1. Solubility of Polymers in Anhydrous and Aqueous Hydrazine
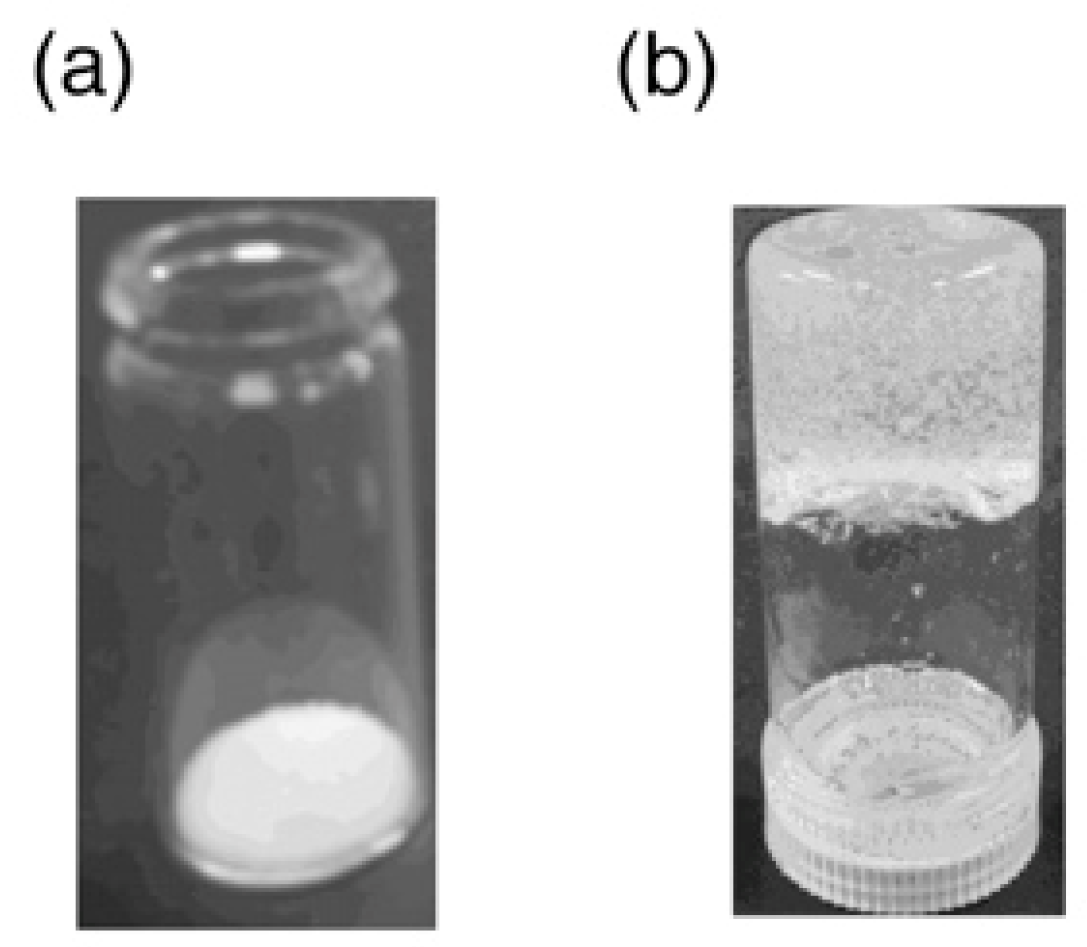
3.2. Gelation of Hydrazine with Cross-Linked Polymers
3.3. Release of Absorbed Hydrazine
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McNutt, R.L., Jr.; Solomon, S.C.; Gold, R.E.; Leary, J.C. The MESSENGER Team, The MESSENGER mission to Mercury: Development history and early mission status. Adv. Space Res. 2006, 38, 564–571. [Google Scholar] [CrossRef]
- Leary, J.C.; Conde, R.F.; Dakermanji, G.; Engelbrecht, C.S.; Ercol, C.J.; Fielhauer, K.B.; Grant, D.G.; Hartka, T.J.; Hill, T.A.; Jaskulek, S.E.; et al. The MESSENGER spacecraft. Space Sci. Rev. 2007, 131, 187–217. [Google Scholar] [CrossRef]
- Jain, S.R. Self-igniting Fuel-oxidizer Systems and Hybrid Rockets. J. Sci. Ind. Res. 2003, 62, 293–310. [Google Scholar]
- Lai, K.Y.; Zhu, R.S.; Lin, M.C. Why mixtures of hydrazine and dinitrogen tetroxide are hypergolic? Chem. Phys. Lett. 2012, 537, 33–37. [Google Scholar] [CrossRef]
- Solomon, Y.; DeFini, S.J.; Pourpoint, T.L.; Anderson, W.E. Gelled Monomethyl Hydrazine Hypergolic Droplet Investigation. J. Propuls. Power 2013, 29, 79–86. [Google Scholar] [CrossRef]
- Davis, S.M.; Yilmaz, N. Advances in Hypergolic Propellants: Ignition, Hydrazine, and Hydrogen Peroxide Research. Adv. Aerosp. Eng. 2014, 2014, 729313. [Google Scholar] [CrossRef]
- Evans, G.E.; Kordesch, K.V. Hydrazine-air fuel cells. Hydrazine-air fuel cells emerge from the laboratory. Science 1967, 158, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Meibuhr, S.G. Surface-Catalyzed Anodes for Hydrazine Fuel Cells I. Preparation of the Substrate. J. Electrochem. Soc. 1974, 121, 1264–1270. [Google Scholar] [CrossRef]
- Demirci, U.B. Direct liquid-feed fuel cells: Thermodynamic and environmental concerns. J. Power Sources 2007, 169, 239–246. [Google Scholar] [CrossRef]
- Asazawa, K.; Yamada, K.; Tanaka, H.; Oka, A.; Taniguchi, M.; Kobayashi, T. A platinum-free zero-carbon-emission easy fuelling direct hydrazine fuel cell for vehicles. Angew. Chem. Int. Ed. 2007, 46, 8024–8027. [Google Scholar] [CrossRef] [PubMed]
- Asazawa, K.; Sakamoto, T.; Yamaguchi, S.; Yamada, K.; Fujikawa, H.; Tanaka, H.; Oguro, K. Study of anode catalysts and fuel concentration on direct hydrazine alkaline anion-exchange membrane fuel cells. J. Electrochem. Soc. 2009, 156, B509–B512. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Direct hydrazine fuel cells: A review. Appl. Catal. B Environ. 2010, 98, 1–9. [Google Scholar] [CrossRef]
- Rees, N.; Compton, R. Carbon-free energy: A review of ammonia- and hydrazine-based electrochemical fuel cells. Energy Environ. Sci. 2011, 4, 1255–1260. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukasawa, K.; Nishino, E.; Yamaguchi, S.; Yamada, K.; Tanaka, H.; Bae, B.; Miyatake, K.; Watanabe, M. Anion conductive block poly(arylene ether)s: Synthesis, properties, and application in alkaline fuel cells. J. Am. Chem. Soc. 2011, 133, 10646–10654. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Chinchilla, J.; Asazawa, K.; Sakamoto, T.; Yamada, K.; Tanaka, H.; Strasser, P. Noble metal-free hydrazine fuel cell catalysts: EPOC effect in competing chemical and electrochemical reaction pathways. J. Am. Chem. Soc. 2011, 133, 5425–5431. [Google Scholar] [CrossRef] [PubMed]
- Granot, E.; Filanovsky, B.; Presman, I.; Kuras, I.; Patolsky, F. Hydrazine/air direct-liquid fuel cell based on nanostructured copper anodes. J. Power Sources 2012, 204, 116–121. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrog. Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Yan, X.; Meng, F.; Xie, Y.; Liu, J.; Ding, Y. Direct N2H4/H2O2 fuel cells powered by nanoporous gold leaves. Sci. Rep. 2012, 2, 941. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhong, X.; Dong, Z.; Wang, J.; Jin, J.; Ma, J. A highly active hydrazine fuel cell catalyst consisting of a Ni-Fe nanoparticle alloy plated on carbon materials by pulse reversal. RSC Adv. 2012, 2, 5038–5040. [Google Scholar] [CrossRef]
- Akbar, K.; Kim, J.; Lee, Z.; Kim, M.; Yi, Y.; Chun, S. Superaerophobic graphene nano-hills for direct hydrazine fuel cells. NPG Asia Mater. 2017, 9, e378. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, G.; Hansen, H. Human health perspective on environmental exposure to hydrazines: A review. Chemosphere 1998, 37, 801–843. [Google Scholar] [CrossRef]
- Ritz, B.; Zhao, Y.; Krishnadasan, A.; Kennedy, N.; Morgenstern, H. Estimated effects of hydrazine exposure on cancer incidence and mortality in aerospace workers. Epidemiology 2006, 17, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Chong, C.H.; Ng, W.T.; Lim, D. Hydrazine inhalation hepatotoxicity. Occup. Med. 2007, 57, 535–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, M.; Gupta, B.L.; Pandey, M. Formulation & storage studies on hydrazine-based gelled propellants. Def. Sci. J. 1996, 46, 435–442. [Google Scholar] [CrossRef]
- Kubota, N.; Fujiyoshi, H.; Ayabe, T. Gelling propellant and manufacture therefor. JP. Patent Application No. 3,876,469, 10 November 2006. (In Japanese). [Google Scholar]
- Rahimi, S.; Hasan, D.; Perets, A. Development of laboratory-scale gel-propulsion technology. J. Propul. Power 2004, 20, 93–100. [Google Scholar] [CrossRef]
- Rahimi, S.; Perets, A.; Natan, B. On share rheology of gel propellants. Propellants Explos. Pyrotech. 2007, 32, 165–174. [Google Scholar] [CrossRef]
- Rahimi, S.; Perets, A.; Natan, B. Rheological Matching of Gel Propellants. J. Propul. Power 2010, 26, 376–379. [Google Scholar] [CrossRef]
- Rahimi, S.; Weihs, D. Gelled fuel simulant droplet impact onto a solid surface. Propellants Explos. Pyrotech. 2011, 36, 273–281. [Google Scholar] [CrossRef]
- Su, X.; Kimura, S.; Wada, M.; Kim, U.-J.; Kuga, S. Complexation of hydrazine with native cellulose in water and toluene. Cellulose 2013, 20, 1023–1029. [Google Scholar] [CrossRef]
- Barton, A.F.M. Handbook of Polymer-Liquid Interaction Parameters and Solubility Parameters; CRC Press: Boca Raton, FL, USA, 1990; ISBN 0-8493-3544-2. [Google Scholar]
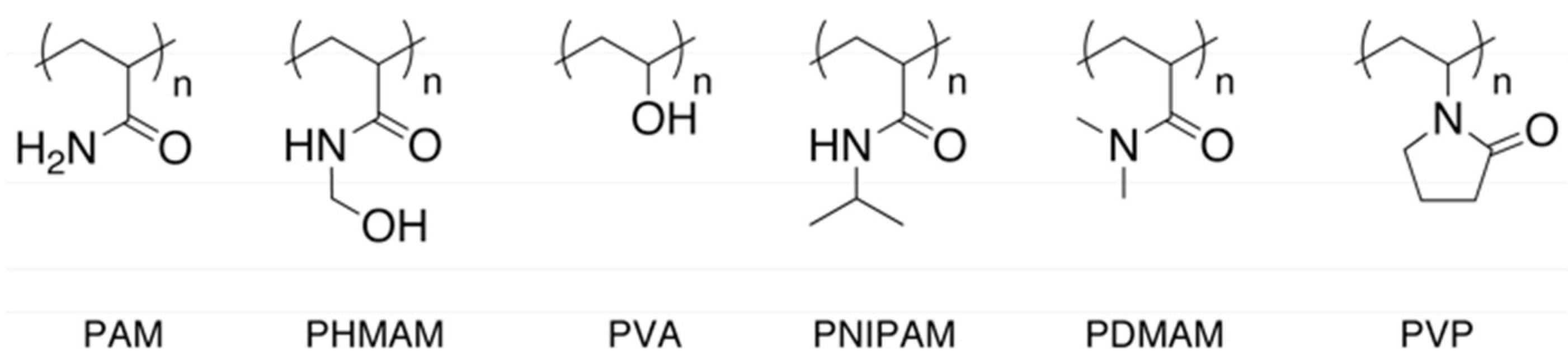


| Polymer (Typical δ in MPa1/2 [31]) | PAM (27–28) | PHMAM (24 or 31) | PVA (26–29) | PNIPAM (22) | PDMAM (18) | PVP (26) |
|---|---|---|---|---|---|---|
| Anhydrous hydrazine | Sol | Sw | Sol | Insol | Insol | Insol |
| 35 wt% hydrazine aq. | Sol | Sw | Sw | Sol below 0 °C | Sol | Sol |
| Absorbent | Anhydrous Hydrazine/Absorbent (w/w) | 35 wt% Hydrazine aq./Absorbent (w/w) |
|---|---|---|
| CPAM | 43/1 | 31/1 |
| CPHMAM | 32/1 | 23/1 |
| CPDMAM | 5/1 | 19/1 |
| CPVA | 10/1 | 5/1 |
| Cycle | Absorbed Hydrazine/CPAM (w/w) | Hydrazine Release Ratio (%) |
|---|---|---|
| 1st | 29/1 | 97 |
| 2nd | 29/1 | 96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiai, B.; Shimada, Y. Reversible Gelation System for Hydrazine Based on Polymer Absorbent. Technologies 2018, 6, 80. https://doi.org/10.3390/technologies6030080
Ochiai B, Shimada Y. Reversible Gelation System for Hydrazine Based on Polymer Absorbent. Technologies. 2018; 6(3):80. https://doi.org/10.3390/technologies6030080
Chicago/Turabian StyleOchiai, Bungo, and Yohei Shimada. 2018. "Reversible Gelation System for Hydrazine Based on Polymer Absorbent" Technologies 6, no. 3: 80. https://doi.org/10.3390/technologies6030080






