Early Autumn Senescence in Red Maple (Acer rubrum L.) Is Associated with High Leaf Anthocyanin Content
Abstract
:1. Introduction


2. Results
2.1. Leaf Chlorophyll Content and Timing of Chlorophyll Degradation
| Variable | Canopy Cover | Soil pH | ||
|---|---|---|---|---|
| r | p | r | p | |
| Chlorophyll degradation | 0.38 | 0.050 * | −0.54 | 0.004 ** |
| Anthocyanin | 0.44 | 0.021 * | −0.46 | 0.017 * |
| Summer chlorophyll | 0.41 | 0.036 * | −0.40 | 0.038 * |
| Tannins | 0.22 | 0.268 | −0.40 | 0.039 * |
| SLA | −0.83 | <0.001 *** | 0.34 | 0.087 |
| DMC | 0.54 | 0.003 ** | −0.51 | 0.007 ** |
| Lamina thickness | 0.66 | <0.001 *** | 0.02 | 0.907 |
| Chlorophyll degradation | 0.38 | 0.050 * | −0.54 | 0.004 ** |

2.2. Leaf Anthocyanin Content
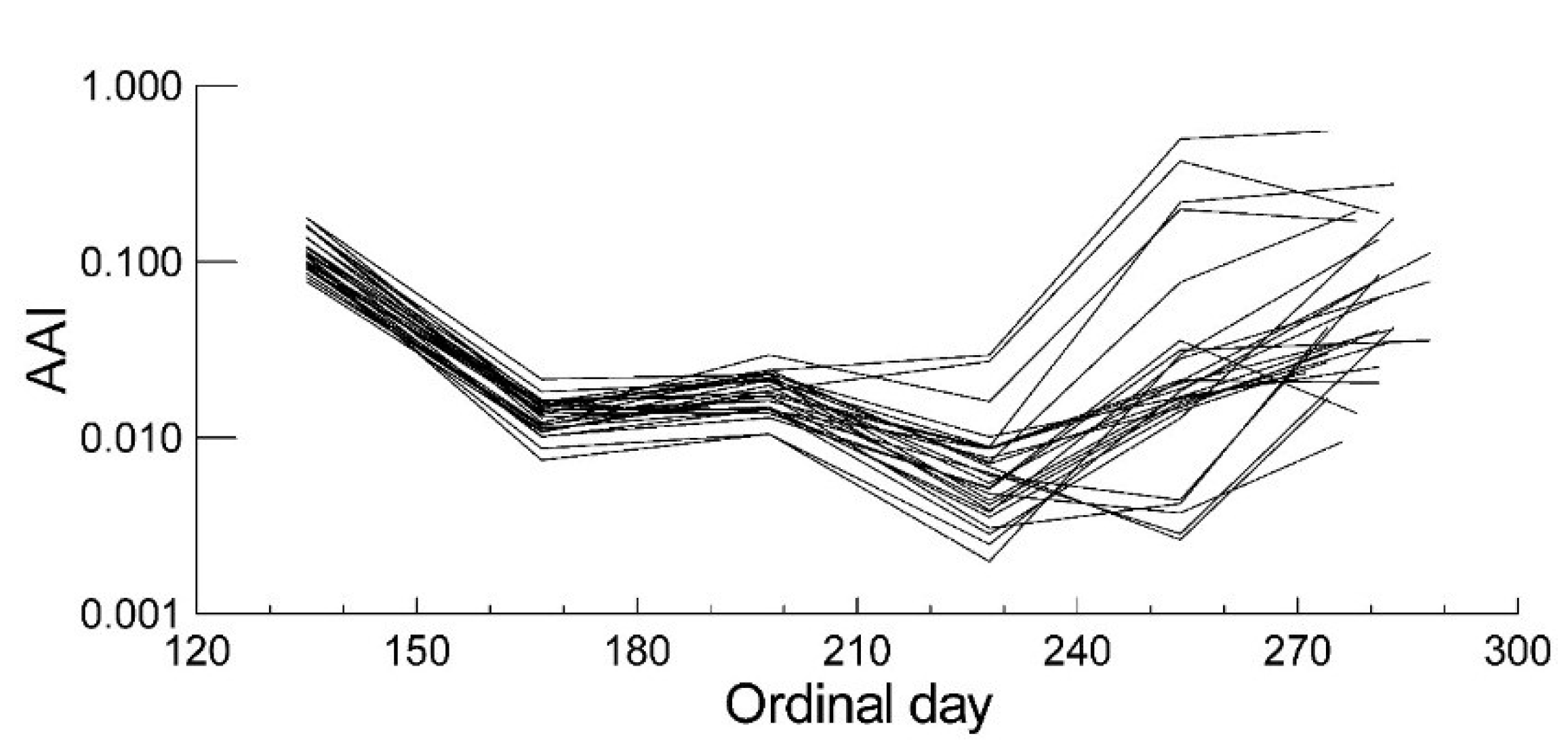
| Anthocyanins | Senescence | |||
|---|---|---|---|---|
| r | p | r | p | |
| Tree height | 0.131 | 0.515 | 0.361 | 0.064 |
| Anthocyanins in spring | −0.367 | 0.060 | 0.147 | 0.465 |
| Chlorophyll | 0.469 | 0.014 * | 0.254 | 0.200 |
| Tannins | 0.360 | 0.065 | 0.274 | 0.167 |
| SLA | −0.439 | 0.022 * | −0.354 | 0.070 |
| DMC | 0.419 | 0.030 * | 0.309 | 0.116 |
| Lamina | 0.294 | 0.137 | 0.303 | 0.124 |
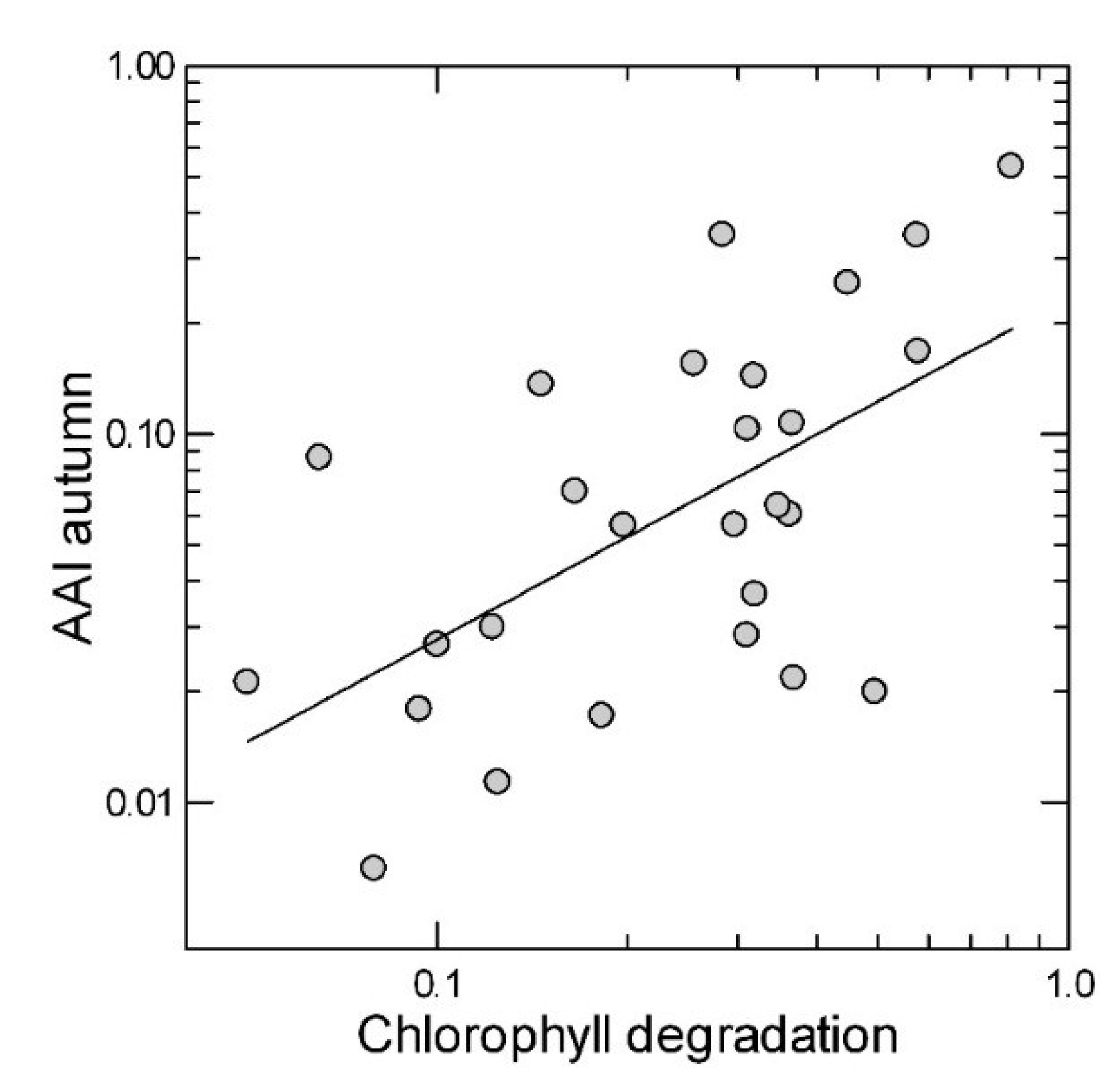
2.3. Leaf Traits in the Summer
3. Discussion
4. Experimental Section
4.1. Plant Material and Measured Variables
4.2. Leaf Collection
4.3. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix

| Tree # | AAI Fall | AAI May May | Senescence Degradation | Chl. Chlorophyll | Tannins | SLA | DMC | Lamina | Height | Canopy | Soil pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.53 | 0.12 | 0.81 | 38 | 0.031 | 12.9 | 0.45 | 0.24 | 9 | 29 | 4.4 |
| 2 | 0.35 | 0.09 | 0.58 | 32 | 0.030 | 14.0 | 0.45 | 0.20 | 9 | 53 | 4.2 |
| 3 | 0.04 | 0.10 | 0.32 | 39 | 0.014 | 13.2 | 0.44 | 0.21 | 10 | 50 | 4.7 |
| 4 | 0.02 | 0.10 | 0.37 | 30 | 0.020 | 19.4 | 0.39 | 0.17 | 7 | 23 | 4.8 |
| 5 | 0.26 | 0.09 | 0.45 | 40 | 0.024 | 13.4 | 0.44 | 0.21 | 7 | 38 | 4.8 |
| 6 | 0.06 | 0.12 | 0.30 | 42 | 0.022 | 12.8 | 0.46 | 0.22 | 8 | 24 | 4.8 |
| 7 | 0.09 | 0.08 | 0.07 | 36 | 0.021 | 16.8 | 0.41 | 0.20 | 1 | 17 | 4.7 |
| 8 | 0.15 | 0.09 | 0.26 | 36 | 0.013 | 26.1 | 0.37 | 0.16 | 5 | 10 | 4.7 |
| 9 | 0.02 | 0.08 | 0.05 | 35 | 0.025 | 18.5 | 0.42 | 0.18 | 8 | 21 | 6.5 |
| 10 | 0.02 | 0.12 | 0.49 | 31 | 0.015 | 21.6 | 0.37 | 0.21 | 12 | 12 | 5.1 |
| 11 | 0.35 | 0.11 | 0.28 | 37 | 0.027 | 18.2 | 0.40 | 0.20 | 4 | 28 | 5.3 |
| 12 | 0.02 | 0.16 | 0.09 | 34 | 0.012 | 22.3 | 0.38 | 0.18 | 11 | 17 | 5.1 |
| 13 | 0.03 | 0.11 | 0.31 | 39 | 0.023 | 17.6 | 0.41 | 0.19 | 4 | 23 | 4.6 |
| 14 | 0.11 | 0.09 | 0.37 | 39 | 0.023 | 15.8 | 0.41 | 0.20 | 5 | 22 | 4.6 |
| 15 | 0.17 | 0.14 | 0.58 | 36 | 0.022 | 17.8 | 0.45 | 0.18 | 3 | 18 | 4.3 |
| 16 | 0.06 | 0.10 | 0.20 | 34 | 0.017 | 20.3 | 0.40 | 0.20 | 8 | 18 | 5.1 |
| 17 | 0.06 | 0.12 | 0.36 | 34 | 0.017 | 20.8 | 0.40 | 0.17 | 2 | 20 | 5.1 |
| 18 | 0.01 | 0.14 | 0.08 | 26 | 0.015 | 26.0 | 0.37 | 0.16 | 1 | 6 | 6.0 |
| 19 | 0.01 | 0.16 | 0.12 | 29 | 0.020 | 22.9 | 0.39 | 0.18 | 1 | 5 | 5.9 |
| 20 | 0.07 | 0.09 | 0.17 | 31 | 0.015 | 20.5 | 0.39 | 0.17 | 3 | 15 | 5.3 |
| 21 | 0.10 | 0.16 | 0.31 | 35 | 0.019 | 14.6 | 0.41 | 0.22 | 4 | 46 | 4.6 |
| 22 | 0.14 | 0.11 | 0.15 | 36 | 0.016 | 16.4 | 0.41 | 0.19 | 6 | 24 | 5.4 |
| 23 | 0.02 | 0.18 | 0.18 | 30 | 0.023 | 15.0 | 0.42 | 0.22 | 2 | 55 | 5.3 |
| 24 | 0.03 | 0.18 | 0.10 | 32 | 0.026 | 14.8 | 0.43 | 0.21 | 2 | 26 | 5.0 |
| 25 | 0.03 | 0.16 | 0.12 | 40 | 0.012 | 14.1 | 0.42 | 0.22 | 4 | 29 | 5.0 |
| 26 | 0.06 | 0.10 | 0.35 | 33 | 0.010 | 16.0 | 0.38 | 0.24 | 7 | 32 | 6.3 |
| 27 | 0.14 | 0.11 | 0.32 | 35 | 0.010 | 14.6 | 0.39 | 0.25 | 7 | 46 | 6.0 |
References
- Matile, P. Biochemistry of Indian summer: Physiology of autumnal leaf coloration. Exp. Gerontol. 2000, 35, 145–158. [Google Scholar] [CrossRef]
- Wheldale, M. The Anthocyanin pigments of Plants; Cambridge University Press: Cambridge, UK, 1916. [Google Scholar]
- Gould, K.S. Muriel Wheldale Onslow and the rediscovery of anthocyanin function in plants. Rec. Adv. Polyphen. Res. 2010, 2, 206–225. [Google Scholar]
- Ford, P.J. Even plants excrete. Nature 1986, 323, 763. [Google Scholar] [CrossRef]
- Archetti, M. The origin of autumn colours by coevolution. J. Theor. Biol. 2000, 205, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Lev-Yadun, S.; Holopainen, J.K. Why red-dominated autumn leaves in America and yellow-dominated autumn leaves in Northern Europe? New Phytol. 2009, 183, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Hoch, W.A.; Zeldin, E.L.; McGown, B.H. Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiol. 2001, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Feild, T.J.; Lee, D.W.; Holbrook, M.N. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol. 2001, 127, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.O.; Gould, K.S. Anthocyanins in leaves: Light attenuators or antioxidants? Funct. Plant Biol. 2003, 30, 865–873. [Google Scholar] [CrossRef]
- Cooney, L.J.; Schaefer, H.M.; Logan, B.A.; Co, B.; Gould, K.S. Functional significance of anthocyanins in peduncles of Sambucus nigra. Environ. Exp. Bot. 2015. [Google Scholar] [CrossRef]
- Pietrini, F.; Iannelli, M.A.; Massacci, A. Anthocyanin accumulation in the illuminated surface of maize leaves enhances protection from photo-inhibitory risks at low temperature, without further limitation to photosynthesis. Plant Cell Environ. 2002, 25, 1251–1259. [Google Scholar] [CrossRef]
- Hoch, W.A.; Singsaas, E.L.; McCown, B.H. Resorption protection. Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiol. 2003, 133, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Juvany, M.; Müller, M.; Munné-Bosch, S. Photo-oxidative stress in emerging and senescing leaves: A mirror image? J. Exp. Bot. 2013, 64, 3087–3098. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.D.; Krakowski, J. Autumn colours—Nature’s canvas is a silk parasol. The adaptive value of autumn foliage. Davidsonia 2003, 24, 111–131. [Google Scholar]
- Oberbauer, S.F.; Starr, G. The role of anthocyanins for photosynthesis of Alaskan arctic evergreens during snowmelt. Adv. Bot. Res. 2002, 37, 129–145. [Google Scholar] [CrossRef]
- Townsend, A.M.; Wright, J.W.; Kwolek, W.F.; Beineke, W.F.; Lester, D.T.; Mohn, C.A.; Dodge, A.F. Geographic variation in young red maple grown in north central United States. Silvae Genet. 1979, 28, 33–36. [Google Scholar]
- Lee, D.W.; O’Keefe, J.; Holbrook, N.M.; Feild, T.S. Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecol. Res. 2003, 18, 677–694. [Google Scholar] [CrossRef]
- Schaberg, P.G.; van den Berg, A.K.; Murakami, P.F.; Shane, J.B.; Donnelly, J.R. Factors influencing red expression in autumn foliage of sugar maple trees. Tree Physiol. 2003, 23, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Munné-Bosch, S. Linking phosphorus availability with photo-oxidative stress in plants. J. Exp. Bot. 2015, 66, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Little, E.L.; Viereck, L.A. Atlas of United States Trees: Conifers and Important Hardwoods; US Department of Agriculture, Forest Service: Washington, DC, USA, 1971.
- Saeki, I.; Dick, C.W.; Barnes, B.V.; Murakami, N. Comparative phylogeography of red maple (Acer rubrum L.) and silver maple (Acer saccharinum L.): Impacts of habitat specialization, hybridization and glacial history. J. Biogeogr. 2011, 38, 992–1005. [Google Scholar] [CrossRef]
- Abrams, M.D. The red maple paradox. Bioscience 1998, 48, 355–364. Available online: http://www.jstor.org/stable/1313374. [Google Scholar]
- Townsend, A.M.; McIntosh, M.S. Variation among full-sib progenies of red maple in growth, autumn leaf color, and leafhopper injury. J. Environ. Hort. 1993, 11, 75–77. [Google Scholar]
- Murray, J.R.; Hackett, W.P. Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedera helix L. Plant Physiol. 1991, 97, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Rolshausen, G.; Schaefer, H.M. Do aphids paint the tree red (or yellow)—Can herbivore resistance or photoprotection explain colourful leaves in autumn? Plant. Ecol. 2007, 191, 77–84. [Google Scholar] [CrossRef]
- Königer, M.; Harris, G.C.; Kibler, E. Seasonal changes in the physiology of shade leaves of Acer saccharum. J. Plant Physiol. 2000, 157, 627–636. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Saliba-Colombani, V.; Loudet, O.; Belluomo, P.; Moreau, L.; Daniel-Vedele, F.; Morot-Gaudry, J.-F.; Masclaux-Daubresse, C. Leaf yellowing and anthocyanin accumulation are two genetically independent strategies in response to nitrogen limitation in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, J.; Bergquist, G.; Gårdeström, P.; Jansson, S. A cellular timetable of autumn senescence. Plant Physiol. 2005, 139, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Schaberg, P.G.; Murakami, P.F.; Turner, M.R.; Heitz, H.K.; Hawley, G.J. Association of red coloration with senescence of sugar maple leaves in autumn. Trees 2008, 22, 573–578. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Tamm, Ü. Species differences in timing of leaf fall and foliage chemistry modify nutrient resorption efficiency in deciduous temperate forest stands. Tree Physiol. 2005, 25, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.M.; Wright, J.W.; Beineke, W.F.; Guries, R.P.; Mohn, C.A. Early patterns of flowering, winter injury, and flushing of red maple progenies grown in five locations. Can. J. For. Res. 1982, 12, 814–821. [Google Scholar] [CrossRef]
- Schröder, F.G. Lehrbuch der Pflanzengeographie; Quelle & Meyer: Wiesbaden, Germany, 1998. [Google Scholar]
- Kumar, L.; Skidmore, A.K.; Knowles, E. Modelling topographic variation in solar radiation in a GIS environment. Int. J. Geog. Inf. Sci. 1997, 11, 475–497. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Government of Canada, Canadian Climate Normals. Available online: http://climate.weather.gc.ca/climate_normals/index_e.html#1981 (accessed on 21 June 2015).
- Nikiforou, C.; Nikolopoulos, D.; Manetas, Y. The winter-red-leaf syndrome in Pistacia lentiscus: Evidence that the anthocyanic phenotype suffers from nitrogen deficiency, low carboxylation efficiency and high risk of photoinhibition. J. Plant. Phys. 2011, 168, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Schachtschabel, P.; Blume, H.P.; Brümmer, G.; Hartge, K.H.; Schwertmann, U. Lehrbuch der Bodenkunde, 12th ed.; Ferdinand Enke Verlag: Stuttgart, Germany, 1989. [Google Scholar]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Peters, D.J.; Constabel, C.P. Molecular analysis of herbivore-induced condensed tannin synthesis: Cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J. 2002, 32, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Hughes, N.M.; Morley, C.B.; Smith, W.K. Coordination of anthocyanin decline and photosynthetic maturation in juvenile leaves of three deciduous tree species. New Phytol. 2007, 175, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Merzlyak, M.N.; Chivkunova, O.B.; Solovchenko, A.E.; Naqvi, K.R. Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J. Exp. Bot. 2008, 59, 3903–3911. [Google Scholar] [CrossRef] [PubMed]
- Lev-Yadun, S.; Yamazaki, K.; Holopainen, J.K.; Sinkkonen, A. Spring versus autumn leaf colours: Evidence for different selective agents and evolution in various species and floras. Flora 2012, 207, 80–85. [Google Scholar] [CrossRef]
- Manetas, Y. Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora 2006, 201, 163–177. [Google Scholar] [CrossRef]
- Hughes, N.M. Winter leaf reddening in “evergreen” species. New Phytol. 2011, 190, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Hughes, N.M.; Neufeld, H.S.; Burkey, K.O. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol. 2005, 168, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Yang, Q.-Y.; Lee, D.W.; Goldstein, G.; Cao, K.F. Extended leaf senescence promotes carbon gain and nutrient resorption: importance of maintaining winter photosynthesis in subtropical forests. Oecologia 2013, 173, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Habinck, E.M.; Eppes, M.C. Correlation of soil development and landscape position with fall leaf colors. In Geological Society of America Abstracts with Programs 39, Proceedings of the GSA Denver Annual Meeting, 28–31 October 2007; p. 218.
- Ryser, P.; Bernardi, J.; Merla, A. Determination of leaf fresh mass after storage between moist paper towels: Constraints and reliability of the method. J. Exp. Bot. 2008, 59, 2461–2467. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Hagerman, A.E. Radial diffusion method for determining tannin in plant extracts. J. Chem. Ecol. 1986, 13, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada, Climate, Daily Data Reports. Available online: http://climate.weather.gc.ca/index_e.html#access (accessed on 23 July 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, R.; Ryser, P. Early Autumn Senescence in Red Maple (Acer rubrum L.) Is Associated with High Leaf Anthocyanin Content. Plants 2015, 4, 505-522. https://doi.org/10.3390/plants4030505
Anderson R, Ryser P. Early Autumn Senescence in Red Maple (Acer rubrum L.) Is Associated with High Leaf Anthocyanin Content. Plants. 2015; 4(3):505-522. https://doi.org/10.3390/plants4030505
Chicago/Turabian StyleAnderson, Rachel, and Peter Ryser. 2015. "Early Autumn Senescence in Red Maple (Acer rubrum L.) Is Associated with High Leaf Anthocyanin Content" Plants 4, no. 3: 505-522. https://doi.org/10.3390/plants4030505
APA StyleAnderson, R., & Ryser, P. (2015). Early Autumn Senescence in Red Maple (Acer rubrum L.) Is Associated with High Leaf Anthocyanin Content. Plants, 4(3), 505-522. https://doi.org/10.3390/plants4030505






