Cellular Auxin Transport in Algae
Abstract
:1. Introduction
2. The Roles of Auxin in Algae
| Kingdom | Division | Class | Order |
|---|---|---|---|
| Plantae | Chlorophyta (green algae) | ||
| Streptophyta | Charophyceae (green algae) | Zygnematales, Coleochaetales, Charales, Desmidiales, Klebsormidiales, Mesostigmatales | |
| Embryophyceae (land plants:mosses lato sensu, lycophytes, ferns and horsetails, seed plants) | |||
| Rhodophyta (red algae) | |||
| Chromalveolata | Heterokontophyta | Phaeophyceae (brown algae) | |
3. Transport of Auxin in Algae
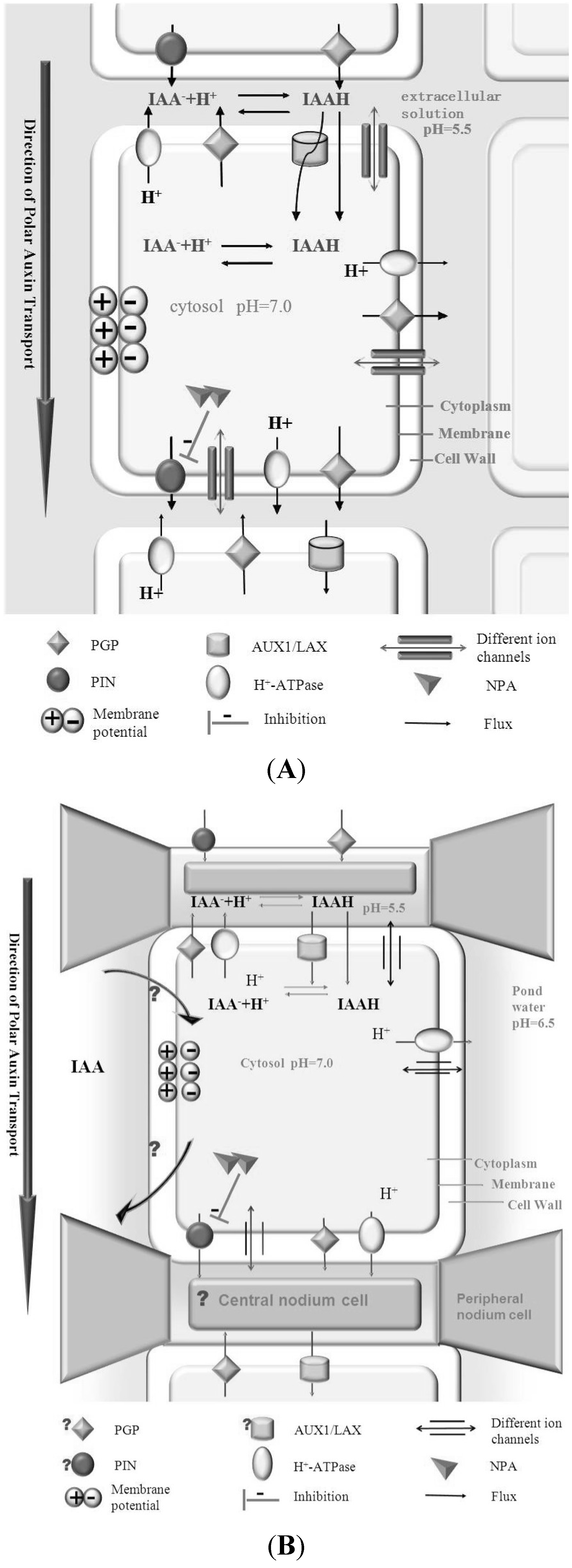
4. Algae as Model System
5. Conclusions
Acknowledgements
Conflicts of Interest
Reference
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants, 6th ed.; W. H. Freeman: New York, NY, USA, 1999; pp. 673–700. [Google Scholar]
- Sslisbury, F.B.; Ross, C.W. Plant Physiology, 4th ed.; Wadsworth Publishing Company: Belmont, CA, USA, 1992; pp. 375–378. [Google Scholar]
- Darwin, C.; Darwin, F. The Power of Movement in Plants; D. Appleton and Company: New York, NY, USA, 1881; pp. 523–545. [Google Scholar]
- Went, F.W. Reflections and speculations. Annu. Rev. Plant Physiol. 1974, 25, 1–26. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; The Benjamin/Cummings Publishing Company, Inc.: Redwood, CA, USA, 1991; pp. 398–425. [Google Scholar]
- Went, F.W.; Thimann, K.V. Phytohormones; The Macmillan Company: New York, NY, USA, 1937; pp. 21–56. [Google Scholar]
- Went, F.W. Growth hormones in the higher plants. Annu. Rev. Biochem. 1939, 8, 521–540. [Google Scholar] [CrossRef]
- Jacobs, W.P. Movement of Hormones. In Plant Hormones and Plant Development; Cambridge University Press: New York, NY, USA, 1979; pp. 195–243. [Google Scholar]
- Lau, S.; Shao, N.; Bock, R.; Jurgens, G.; Smet, I.D. Auxin signaling in algal lineages: Fact or myth? Trends Plant Sci. 2009, 14, 182–188. [Google Scholar] [CrossRef]
- Friml, J. Auxin transport-shaping the plant. Curr. Opin. Plant Biol. 2003, 6, 7–12. [Google Scholar] [CrossRef]
- Friml, J.; Palme, K. Polar auxin transport—Old questions and new concepts? Plant Mol. Biol. 2002, 49, 273–284. [Google Scholar] [CrossRef]
- Bartel, B. Auxin biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 51–66. [Google Scholar] [CrossRef]
- Vieten, A.; Sauer, M.; Brewer, P.B.; Friml, J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007, 12, 160–168. [Google Scholar] [CrossRef]
- Petrasek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef]
- Ergun, N.; Topcuoglu, S.F.; Yildiz, A. Auxin (indole-3-acetic acid), Gibberellic acid (GA3), Abscisic Acid (ABA) and Cytokinin (Zeatin) production by some species of mosses and lichens. Turk. J. Bot. 2002, 26, 13–18. [Google Scholar]
- Van Overbeek, J. Auxin in marine algae. Plant Physol. 1940, 15, 291–299. [Google Scholar] [CrossRef]
- Cooke, T.J.; Poli, D.B.; Sztein, A.E.; Cohen, J.D. Evolutionary patterns in auxin action. Plant Mol. Biol. 2002, 49, 319–338. [Google Scholar] [CrossRef]
- Proseus, T.E.; Boyer, J.S. Identifying cytoplasmic input to the cell wall of growing Chara coralline. J. Exp. Bot. 2006, 57, 3231–3242. [Google Scholar] [CrossRef]
- Niklas, K.J. The Evolution of plant body plans—A biomechanical perspective. Ann. Bot. 2000, 85, 411–438. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Niemann, D.I.; Dorffling, K. Growth inhibitors and growth promoters in Enteromorpha compressa (Chlorophyta). J. Phycol. 1980, 16, 383–389. [Google Scholar] [CrossRef]
- Jacobs, W.P.; Falkenstein, K.; Hamilton, R.H. Nature and amount of auxin in algae. Plant Physiol. 1985, 78, 844–848. [Google Scholar] [CrossRef]
- Kenneth, V.T.; Kurt, B. Action of auxins on Acetabulatia and the effect of enucleation. Nature 1959, 183, 946–948. [Google Scholar] [CrossRef]
- Nowak, J.; Sonaike, B.; Lawson, G.W. Auxin induced stress tolerance in algae. Environ. Pollut. 1988, 51, 213–218. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Handro, W. Effects of auxins and cytokinins on tissue culture of Grateloupia dichotoma (Gigartinales, Rhodophyta). Hydrobologia 1996, 326/327, 393–400. [Google Scholar] [CrossRef]
- Garcia-Jimenez, P.; Rodrigo, M.; Robaina, R.R. Influence of plant growth regulators, Polyamines and Glycerol interaction on growth and morphogenesis of carposporelings of Grateloupia cultured in vitro. J. Appl. Phycol. 1998, 10, 95–100. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Hirotaka, K.; Hideki, O.; Takao, K. Effects of environmental factors and plant growth regulators on growth of the red alga Gracilaria vermiculophylla from Shikoku Island, Japan. Hydrobiologya 1999, 398/399, 339–347. [Google Scholar] [CrossRef]
- Vance, B.D. Phytohormone effects on cell division in Chlorella pyrenoidosa chick (TX-7-11-05) (Chlorellaceae). J. Plant Growth Regul. 1987, 5, 169–173. [Google Scholar] [CrossRef]
- Wood, N.L.; Berliner, M.D. Effects of indoleacetic acid on the desmid Micrasterias thomasiana. Plant Sci. Lett. 1979, 16, 285–289. [Google Scholar] [CrossRef]
- Bradley, P.M.; Cheney, D.P. Some effects of plant growth regulators on tissue cultures of the marine red alga Agardhiella subulata (Gigartinales, Rhodophyta). Hydrobiologia 1990, 204/205, 353–360. [Google Scholar] [CrossRef]
- Basu, S.; Haiguo, S.; Brian, L.; Quatrano, R.L.; Muday, G.K. Early embryo development in Fucus distichus is auxin sensitive. Plant Physiol. 2002, 130, 292–302. [Google Scholar] [CrossRef]
- Klambt, D.; Knauth, B.; Dittmann, I. Auxin dependent growth of rhizoids of Chara globularis. Physiol. Plant. 1992, 85, 537–540. [Google Scholar] [CrossRef]
- Provasoli, L.; Carlucci, A.F. Vitamins and Growth Regulators. In Algal Physiology and Biochemistry; Stewart, W.D.P., Ed.; Blackwell Scientific Publications Ltd: Oxford, UK, 1974; pp. 741–787. [Google Scholar]
- Bradley, P.M. Plant hormones do have a role in controlling growth and development of algae. J. Phycol. 1991, 27, 317–321. [Google Scholar]
- Evans, L.V.; Trewavas, A.J. Is algal development controlled by plant growth substances? J. Phycol. 1991, 27, 322–326. [Google Scholar]
- Buggeln, R.G. Morphogenesis and Growth Regulators. In The Biology of Seaweeds; Lobban, C.S., Wynne, M.J., Eds.; University of California press: Berkeley, CA, USA, 1981; pp. 627–660. [Google Scholar]
- Smet, I.D.; Voß, U.; Lau, S.; Wilson, M.; Shao, N.; Timme, R.E.; Swarup, R.; Kerr, I.; Hodgman, C.; Bock, R.; et al. Unraveling the evolution of auxin signaling. Plant Physiol. 2011, 155, 209–221. [Google Scholar] [CrossRef]
- Feraru, E.; Vosolsobe, S.; Ferar1, M.I.; Petrášek, J.; Kleine-Vehn, J. Evolution and structural diversification of PILS putative auxin carriers in plants. Front. Plant Sci. 2012, 3, 1–13. [Google Scholar]
- Lomax, T.L.; Muday, G.K.; Rubery, P.H. Auxin Transpor. In Plant Hormones: Physiology, Biochemistry, and Molecular Biology, 2nd ed.; Davies, P.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 509–530. [Google Scholar]
- Morris, D.A. Transmembrane auxin carrier systems—Dynamic regulators of polar auxin transport. Plant Growth Regul. 2000, 32, 161–172. [Google Scholar] [CrossRef]
- Paciorek, T.; Zazimalova, E.; Ruthardt, N.; Petrasek, J.; Stierhof, Y.D.; Kleine-Vehn, J.; Morris, D.A.; Emans, N.; Jurgens, G.; Geldner, N.; et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 2005, 435, 1251–1256. [Google Scholar] [CrossRef]
- Friml, J. Subcellular trafficking of PIN auxin efflux carriers in auxin transport. Eur. J. Cell Biol. 2010, 89, 231–235. [Google Scholar] [CrossRef]
- Barbez, E.; Kubes, M.; Rolcik, J.; Beziat, C.; Pencik, A.; Wang, B.; Rosquete, M.R.; Zhu, J.; Dobrev, P.I.; Lee, Y.; et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 2012, 485, 119–122. [Google Scholar] [CrossRef]
- Mravec, J.; Skupa, P.; Bailly, A.; Hoyerova, K.; Krecek, P.; Bielach, A.; Petrasek, J.; Zhang, J.; Gaykova, V.; Stierhof, Y.; et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 2009, 495, 1136–1140. [Google Scholar]
- Sauer, M.; Robert, S.; Kleine-Vehn, J. Auxin: Simply complicated. J. Exp. Bot. 2013, 64, 2565–2577. [Google Scholar] [CrossRef]
- Dibb-Fuller, J.E.; Morris, D.A. Studies on the evolution of auxin carriers and phytotropin receptors: Transmembrane auxin transport in unicellular and multicellular Chlorophyta. Planta 1992, 186, 219–226. [Google Scholar]
- Brennan, T.; Jacobs, W.P. Polarity and the movement of [14C]-indol-3-ylacetic acid in the coenocyte, Caulerpa prolifera. Ann. Bot. 1980, 46, 129–131. [Google Scholar]
- Boot, K.J.M.; Libbenga, K.R.; Hille, S.C.; Offringa, R.; van Duijn, B. Polar auxin transport: An early invention. J. Exp. Bot. 2012, 63, 4213–4218. [Google Scholar] [CrossRef]
- Sun, H.; Basu, S.; Brady, S.R.; Luciano, R.L.; Muday, G.K. Interactions between auxin transport and the actin cytoskeleton in developmental polarity of Focus distichus embryos in response to light and gravity. Plant Physiol. 2004, 135, 266–278. [Google Scholar] [CrossRef]
- Goldsmith, M.H.M. The polar transport of auxin. Ann. Rev. Plant Physiol. 1977, 28, 439–478. [Google Scholar] [CrossRef]
- Viaene, T.; Delwiche, C.F.; Rensing, S.A.; Friml, J. Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci. 2013, 18, 5–10. [Google Scholar] [CrossRef]
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.D.; Verbruggen, H.; Delwiche, C.F.; de Clerck, O. Phylogeny and molecular evolution of the green algae. CRC Crit. Rev. Plant Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef]
- Bail, A.L.; Billoud, B.; Kowalczyk, N.; Kowalczyk, M.; Gicquel, M.; Panse, S. L.; Stewart, S.; Scornet, D.; Cock, J. M.; Ljung, K.; et al. Auxin metabolism and function in the multicellular Brown alga Ectocarpus siliculosus. Plant Physiol. 2010, 153, 128–144. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Lucas, W.J. Structure and possible function(s) of charasomes; complex plasmalemma-cell wall elaborations present in some Characean species. Protoplasma 1980, 104, 253–271. [Google Scholar] [CrossRef]
- Lucas, W.J.; Franceschi, V.R. Characean charasome-complex and plasmalemma vesicle development. Protoplasma 1981, 107, 255–267. [Google Scholar] [CrossRef]
- Schmolzer, P.M.; Hoftberger, M.; Foissner, I. Plasma membrane domains participate in pH banding of Chara internodal cells. Plant Cell Physiol. 2011, 52, 1274–1288. [Google Scholar] [CrossRef]
- Boot, K.J.M.; Libbenga, K.R.; Offringa, R.; van Duijn, B.; Institute Biology Leiden, Leiden, The Netherlands. Unpublished work. 2003.
- Raven, J.A. Polar auxin transport in relation to long-distance transport of nutrients in the Charales. Exp. Bot. 2013, 64, 1–9. [Google Scholar] [CrossRef]
- Raven, J.A. Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol. 1975, 74, 163–172. [Google Scholar] [CrossRef]
- Rubby, P.G.; Sheldrake, A.R. Effect of pH and surface charge on cell uptake of auxin. Nat. New Biol. 1973, 139, 285–288. [Google Scholar]
- Fisahn, J.; Hansen, U.P.; Lucas, W.J. Reaction kinetic model of a proposed plasma membrane two-cycle H+-transport system of Chara corallina. Proc. Natl. Acad. Sci. USA 1992, 89, 3261–3265. [Google Scholar] [CrossRef]
- Karol, K.G.; McCourt, R.M.; Cimino, M.T.; Delwiche, C.F. The closest living relatives of land plants. Science 2001, 294, 2351–2353. [Google Scholar] [CrossRef]
- Fujita, T.; Hasebe, M. Convergences and divergences in polar auxin transport and shoot development in land plant evolution. Plant Signal. Behav. 2009, 4, 313–315. [Google Scholar] [CrossRef]
- Finet, C.; Timme, R.E.; Delwiche, C.F.; Marletaz, F. Multigene phylogeny of the green lineage reveals the origin and diversification of land plants. Curr. Biol. 2010, 20, 2217–2222. [Google Scholar] [CrossRef]
- Wodniok, S.; Brinkmann, H.; Glockner, G.; Heidel, A.J.; Philippe, H.; Melkonian, M.; Becker, B. origin of land plants: Do conjugating green algae hold the key? BMC Evol. Biol. 2011, 11, e104. [Google Scholar] [CrossRef]
- Timme, R.E.; Bachvaroff, T.R.; Delwiche, C.F. Broad phylogenomic sampling and the sister lineage of land plants. PLoS One 2012, 7, e29696. [Google Scholar]
- Morris, D.A.; Johnson, C.F. The role of auxin efflux carriers in the reversible loss of polar auxin transport in the pea (Pisum sativum L.) stem. Planta 1990, 181, 117–124. [Google Scholar]
- Lucas, W.J. Alkaline band formation in Chara corallina. Plant Physiol. 1979, 63, 248–254. [Google Scholar] [CrossRef]
- Lucas, W.J. Plasmalemma transport of OH– in Chara corallina III. Further studies on transport substrate and directionality. Plant Physiol. 1980, 66, 46–50. [Google Scholar] [CrossRef]
- Shimmen, T.; Wakabayashi, A. Involvement of membrane potential in alkaline band formation by internodal cells of Chara corallina. Plant Cell Physiol. 2008, 49, 1614–1620. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, S.; Van Duijn, B. Cellular Auxin Transport in Algae. Plants 2014, 3, 58-69. https://doi.org/10.3390/plants3010058
Zhang S, Van Duijn B. Cellular Auxin Transport in Algae. Plants. 2014; 3(1):58-69. https://doi.org/10.3390/plants3010058
Chicago/Turabian StyleZhang, Suyun, and Bert Van Duijn. 2014. "Cellular Auxin Transport in Algae" Plants 3, no. 1: 58-69. https://doi.org/10.3390/plants3010058




