Pollen Tube Growth and Self-Compatibility in Almond
Abstract
:1. Introduction
2. Results and Discussion
| SC index | SC classification | PTG score | Genotypes |
|---|---|---|---|
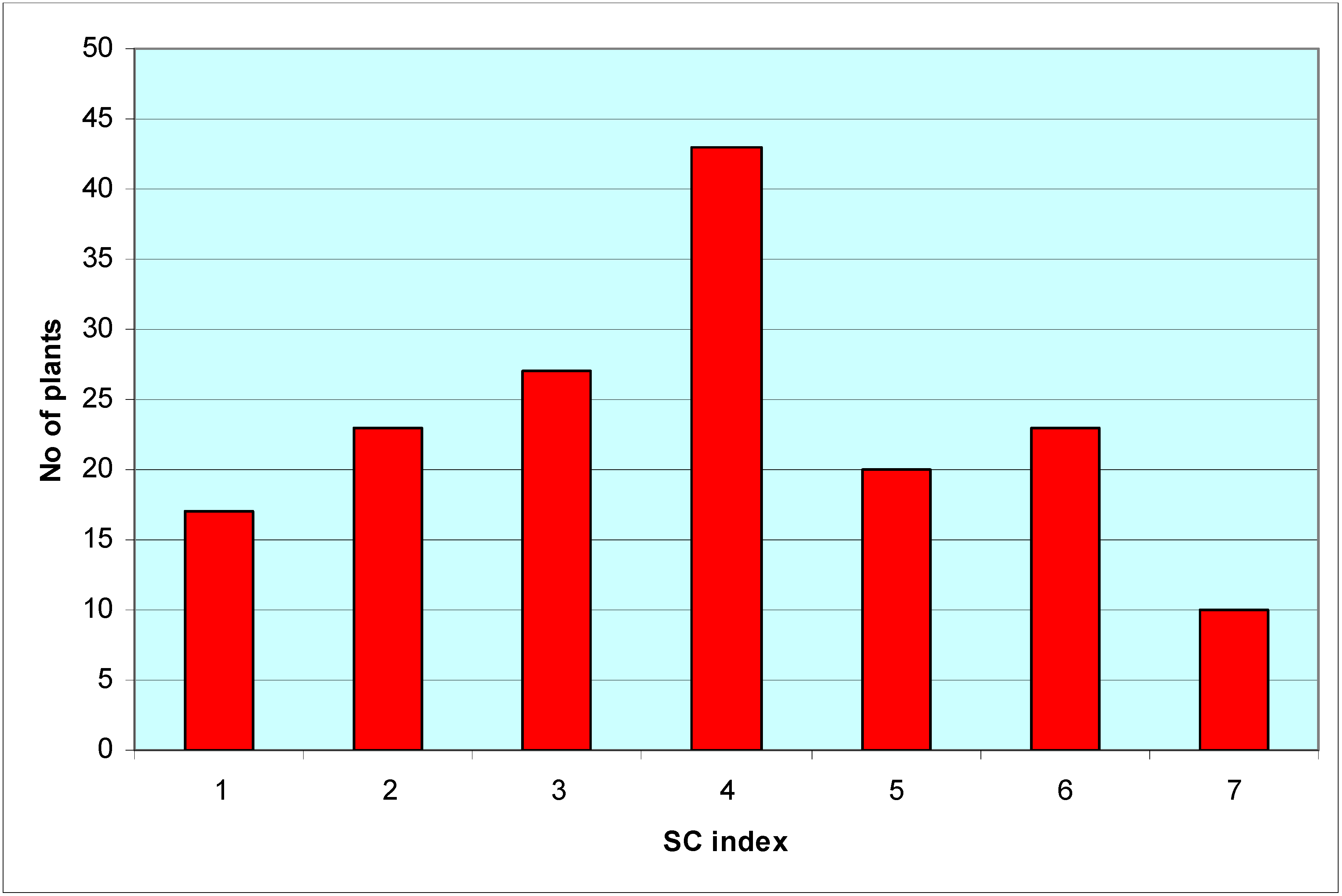
| 1 | Fully SI | <50.1 | P-7-68, P-7-49, P-7-53, P-7-60, P-7-76, P-7-82, P-7-87,P-7-92, P-8-14, P-8-27, P-8-32, P-8-69, P-8-70, P-8-72,P-8-86, P-8-87, P-8-98 |
| 2 | SI | 50.1–60 | P-7-51, P-8-83, P-7-38, P-7-86, P-8-57, P-8-77, P-8-99,P-8-90, P-8-25, P-8-68, P-7-48, P-8-9, P-8-97, P-7-67,P-7-42, P-8-19, P-7-50, P-8-48, P-7-95, P-8-78, P-7-62,P-8-74, P-7-91 |
| 3 | SI doubtful | 60.1–70 | P-7-61, P-7-69, P-8-89, P-8-62, P-8-34, P-8-43, P-8-23,P-8-16, P-8-91, P-8-81, P-8-18, P-7-74, P-7-45, P-7-43,P-8-28, P-7-36, P-8-45, P-7-41, P-8-94, P-7-81, P-8-8,P-7-37, P-7-64, P-8-39, P-8-20, P-7-75, P-8-6 |
| 4 | Doubtful | 70.1–80 | P-8-13, P-7-90, P-8-61, P-8-17, P-8-71, P-8-40, P-7-96,P-7-98, P-7-52, P-8-11, P-7-44, P-8-73, P-7-89, P-8-76,P-8-5, P-7-97, P-8-24, P-8-51, P-8-56, P-7-73, P-8-96,P-8-49, P-7-65, P-8-26, P-7-80, P-8-42, P-7-59, P-8-59,P-8-82, P-8-80, P-8-84, P-7-79, P-8-35, P-8-4, P-8-55,P-8-92, P-8-38, P-7-70, P-8-22, P-8-47, P-8-12, P-7-57,P-8-15 |
| 5 | SC doubtful | 80.1–90 | P-7-78, P-8-54, P-8-46, P-7-85, P-8-7, P-8-95, P-8-2,P-8-41, P-8-88, P-8-30, P-8-66, P-8-67, P-8-31, P-8-75,P-7-40, P-8-93, P-8-50, P-7-94, P-7-84, P-7-63 |
| 6 | SC | 90.1–99.9 | P-8-58, P-8-85, P-8-53, P-8-10, P-8-29, P-8-52, P-8-65,P-8-60, P-7-39, P-7-56, P-7-83, P-8-1, P-8-79, P-7-35,P-8-63, P-7-47, P-7-71, P-8-36, P-8-3, P-7-46, P-7-77,P-8-44, P-8-64 |
| 7 | Fully SC | 100 | P-7-54, P-7-55, P-7-58, P-7-66, P-7-72, P-7-88, P-7-93,P-7-99, P-8-21, P-8-33 |
3. Experimental Section
| Level of PTG | Rating |
|---|---|
| Pollen tubes at the style base | 100 |
| Pollen tubes near the style base with no signs of incompatibility, thus suggesting that pollen tubes could reach the ovary | 95 |
| Pollen tubes near the style base with signs of incompatibility, thus suggesting that pollen tubes could not reach the ovary | 90 |
| Pollen tubes reaching the middle third of the pistil | 50 |
| Pollen tubes just penetrating the style, but not growing down | 10 |
| Germination of pollen grains but pollen tubes not penetrating the style | 5 |
| Pollen grains on the stigma but no pollen germination | 0 |
| No pollen grains on the stigma | Not included in the analysis |
4. Conclusions
Acknowledgments
References
- Socias i Company, R.; Alonso, J.M.; Kodad, O.; Gradziel, T.M. Almond. In Fruit Breeding; Badenes, M.L., Byrne, D., Eds.; Springer Verlag: Heidelberg, Germany, 2012; pp. 697–728. [Google Scholar]
- Socias i Company, R. Breeding self-compatible almonds. Plant Breed. Rev. 1990, 8, 313–338. [Google Scholar]
- Socias i Company, R.; Kester, D.E.; Bradley, M.V. Effects of temperature and genotype on pollen tube growth of some self-compatible and self-incompatible almond cultivars. J. Amer. Soc. Hort. Sci. 1976, 101, 490–493. [Google Scholar]
- Socias i Company, R.; Felipe, A.J. Pollen tube growth and fruit set in a self-compatible almond selection. HortScience 1987, 22, 113–116. [Google Scholar]
- Socias i Company, R.; Alonso, J.M.; Gómez Aparisi, J. Fruit set and productivity in almond as related to self-compatibility, flower morphology and bud density. J. Hort. Sci. Biotechnol. 2004, 79, 754–758. [Google Scholar]
- Weinbaum, S.A. Role of natural self-pollination in self-fruitfulness in almond. Scientia Hort. 1985, 27, 295–302. [Google Scholar] [CrossRef]
- Socias i Company, R. A genetic approach to the transmission of self-compatibility in almond. Options Méditerr. IAMZ 1984, 84/II, 123–128. [Google Scholar]
- Channuntapipat, C.; Sedgley, M.; Collins, G. Sequences of the cDNAs and genomic DNAs encoding the S1, S7, S8 and Sf alleles from almond, Prunus dulcis. Theor. Appl. Genet. 2001, 103, 1115–1122. [Google Scholar] [CrossRef]
- Ma, R.C.; Oliveira, M.M. The RNase PD2 gene of almond (Prunus dulcis) represents an evolutionary distinct class of S-like RNase genes. Mol. Gen. Genet. 2001, 263, 925–933. [Google Scholar]
- Kodad, O.; Socias i Company, R.; Sánchez, A.; Oliveira, M.M. The expression of self-compatibility in almond may not only be due to the presence of the Sf allele. J. Amer. Soc. Hort. Sci. 2009, 134, 221–227. [Google Scholar]
- Fernández i Martí, A.; Hanada, T.; Alonso, J.M.; Yamane, H.; Tão, R.; Socias i Company, R. A modifier locus affecting the expression of the S-Nase gene could be the cause of breakdown of self-incompatibility in almond. Sex. Plant Reprod. 2009, 22, 179–186. [Google Scholar] [CrossRef]
- Kodad, O.; Alonso, J.M.; Fernández i Martí, A.; Oliveira, M.M.; Socias i Company, R. Molecular and physiological identification of new S-alleles associated with self-(in)compatibility in local Spanish almond cultivars. Scientia Hort. 2010, 123, 308–311. [Google Scholar] [CrossRef]
- Howad, W.; Tao, R.; Alonso, J.M.; Arús, P.; Socias i Company, R. Identification of quantitative trait loci associated with self-compatibility in Prunus. Tree Genet. Genomes 2011, 7, 629–639. [Google Scholar] [CrossRef]
- Socias i Company, R.; Fernández i Martí, À.; Kodad, O.; Alonso, J.M. Self-compatibility evaluation in almond: strategies, achievements and failures. HortScience 2010, 45, 1155–1159. [Google Scholar]
- Socias i Company, R. Differential growth of almond pollen tubes in three environments. Cah. Options Méditerr. 2001, 56, 59–64. [Google Scholar]
- Socias i Company, R. Ensayo de polinización de diferentes clones del almendro “Marcona”. An. Inst. Nac. Invest. Agrar. Ser. Agric. 1982, 19, 11–21. [Google Scholar]
- Ben Njima, N.; Socias i Company, R. Characterization of some self-compatible almonds. I. Pollen tube growth. HortScience 1995, 30, 318–320. [Google Scholar]
- Kodad, O.; Socias i Company, R. Pollen source effect on pollen tube growth in advanced self-compatible almond selections (Prunus amygdalus Batsch). Adv. Hort. Sci. 2006, 20, 256–261. [Google Scholar]
- Alonso, J.M.; Socias i Company, R. Self-compatibility expression in self-compatible almond genotypes may be due to inbreeding. J. Amer. Soc. Hort. Sci. 2005, 130, 868–869. [Google Scholar]
- Socias i Company, R.; Kodad, O.; Alonso, J.M.; Felipe, A.J. “Mardía” almond. HortScience 2008, 43, 2240–2242. [Google Scholar]
- Almeida, C.R. Marques de. Âcerca da improdutividade na amendoeira. An. Inst. Agron. Lisboa 1945, 15, 1–186. [Google Scholar]
- Kodad, O.; Socias i Company, R. Fruit set evaluation for self-compatibility selection in almond. Scientia Hort. 2008, 118, 260–265. [Google Scholar] [CrossRef]
- Socias i Company, R.; Gómez Aparisi, J.; Alonso, J.M. Year and enclosure effects on fruit set in an autogamous almond. Scientia Hort. 2005, 104, 369–377. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Fujimura, M.; Hayashida, T.; Nishikawa, Y.; Nada, K. Pollen factors controlling self-incompatibility strength in Japanese pear. Sex. Plant Reprod. 2012, 25, 347–352. [Google Scholar] [CrossRef]
- Felipe, A.J. Almendro. Estados fenológicos. Inf. Técn. Econ. Agrar. 1977, 27, 8–9. [Google Scholar]
- Socias i Company, R. Aportación a las técnicas de observación de tubos polínicos. Caso del almendro. An. Inst. Nac. Invest. Agrar. Ser. Prod. Veg. 1979, 10, 233–236. [Google Scholar]
- Linskens, M.F.; Esser, K. Über eine spezifische Anfarbung der Pollensläuche und die Zahl der Kallopsepfropfen nach Selbstung und Fremdung. Naturwissenchften 1957, 44, 16. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Socias i Company, R.; Kodad, O.; Fernández i Martí, À.; Alonso, J.M. Pollen Tube Growth and Self-Compatibility in Almond. Plants 2013, 2, 50-56. https://doi.org/10.3390/plants2010050
Socias i Company R, Kodad O, Fernández i Martí À, Alonso JM. Pollen Tube Growth and Self-Compatibility in Almond. Plants. 2013; 2(1):50-56. https://doi.org/10.3390/plants2010050
Chicago/Turabian StyleSocias i Company, Rafel, Ossama Kodad, Àngel Fernández i Martí, and José M. Alonso. 2013. "Pollen Tube Growth and Self-Compatibility in Almond" Plants 2, no. 1: 50-56. https://doi.org/10.3390/plants2010050




