Antioxidant Activities and Anti-Cancer Cell Proliferation Properties of Natsuhaze (Vaccinium oldhamii Miq.), Shashanbo (V. bracteatum Thunb.) and Blueberry Cultivars
Abstract
:1. Introduction
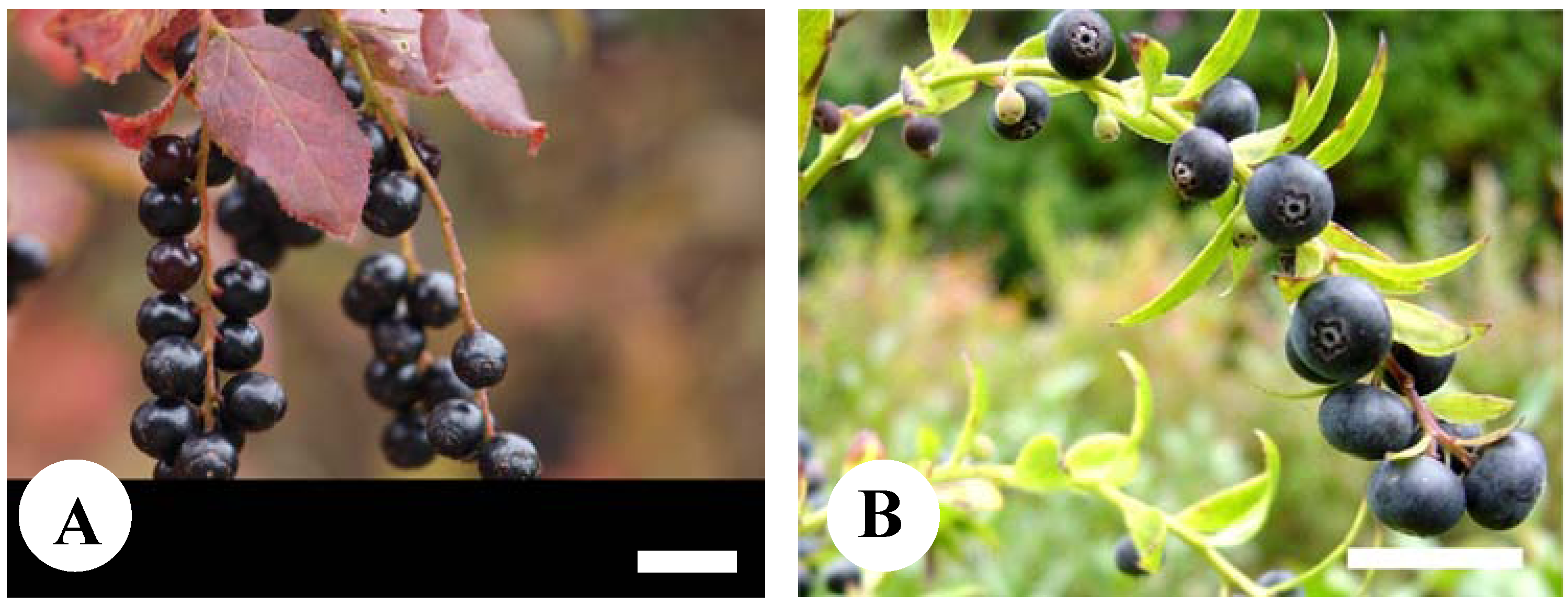
2. Results
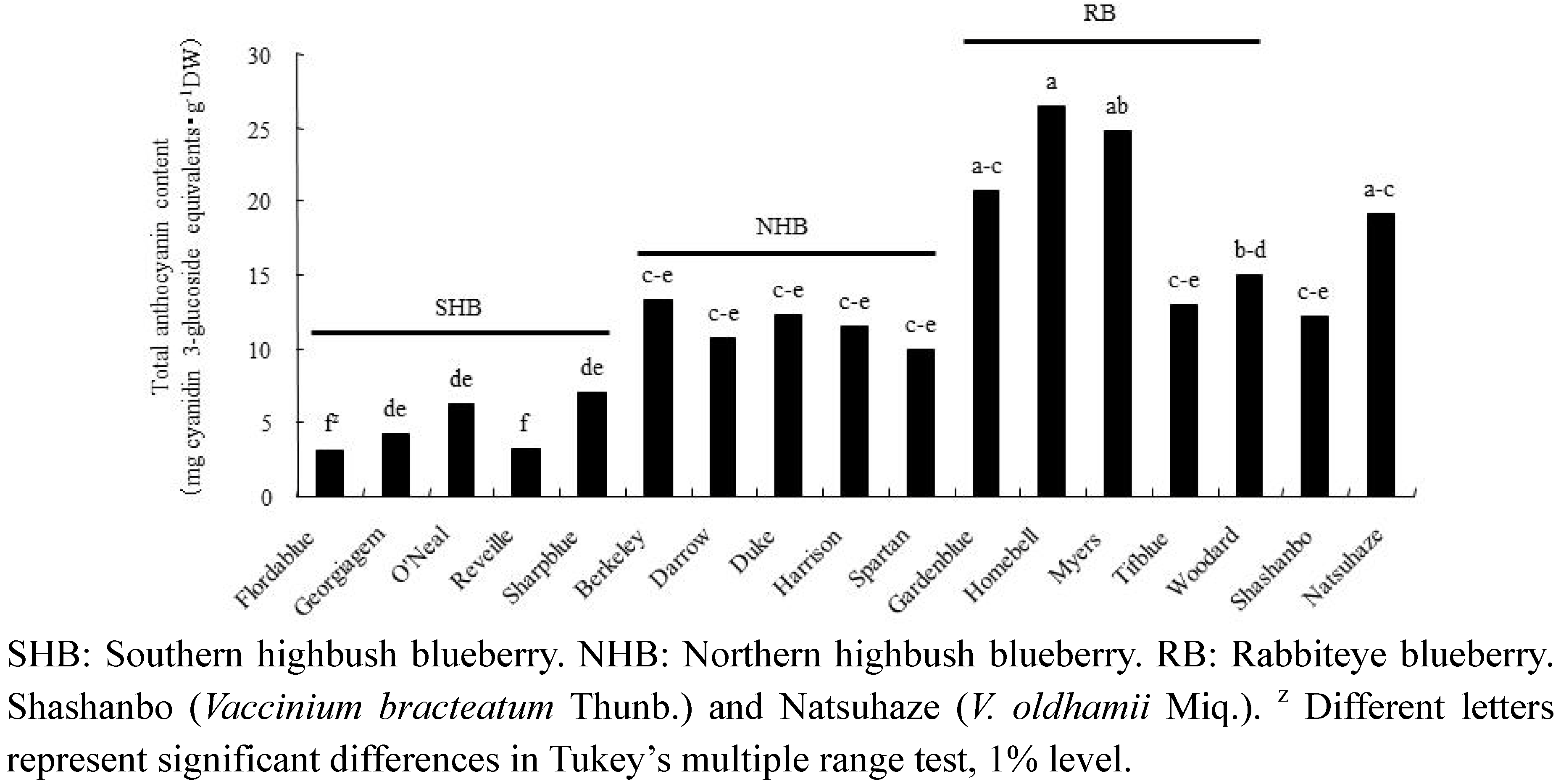
2.1. Anthocyanins
2.2. Total Polyphenol

2.3. Antioxidant Activity

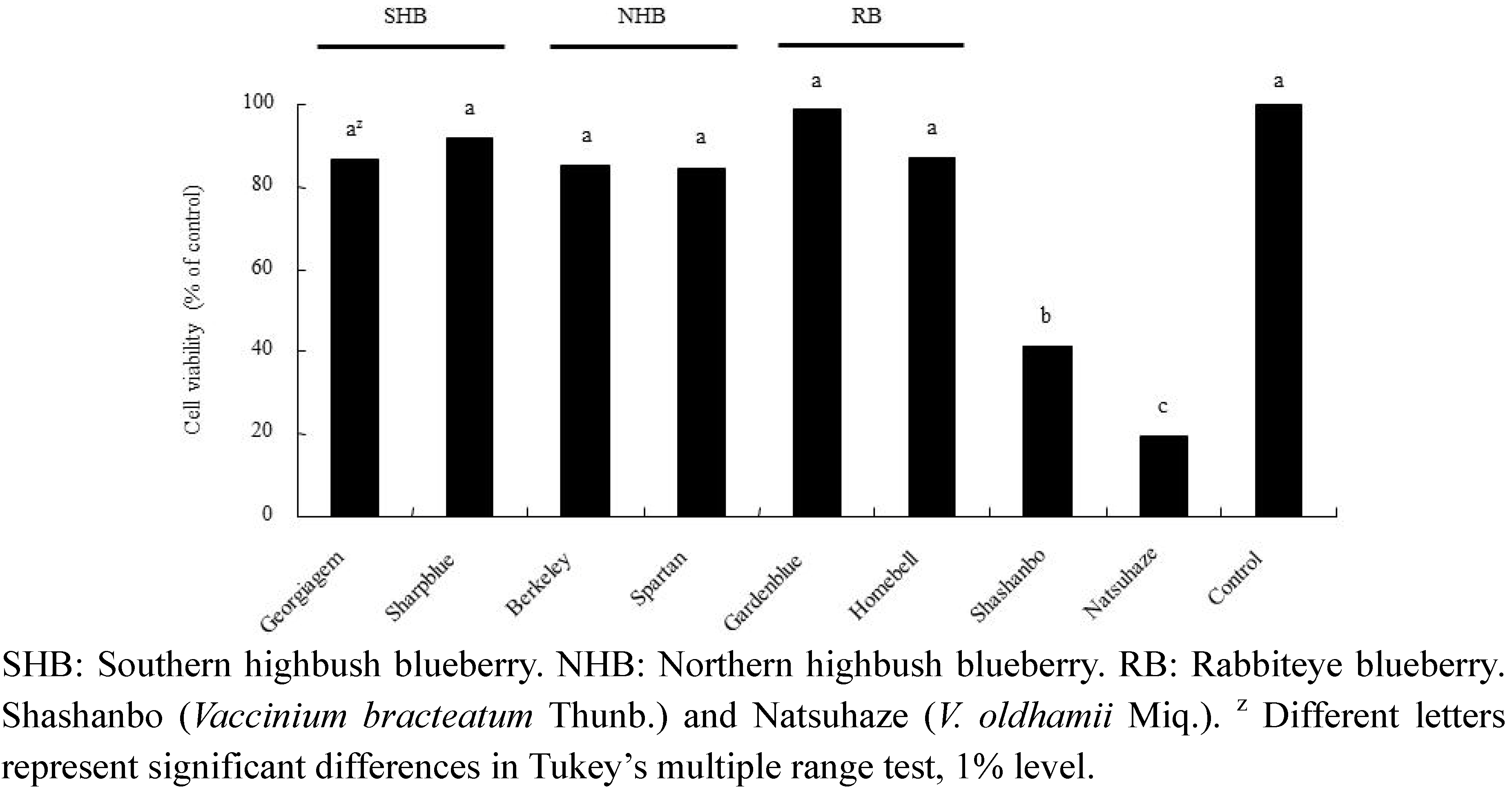
2.4. Growth Inhibitory Effect of Fruit Extracts on HL-60 Cells
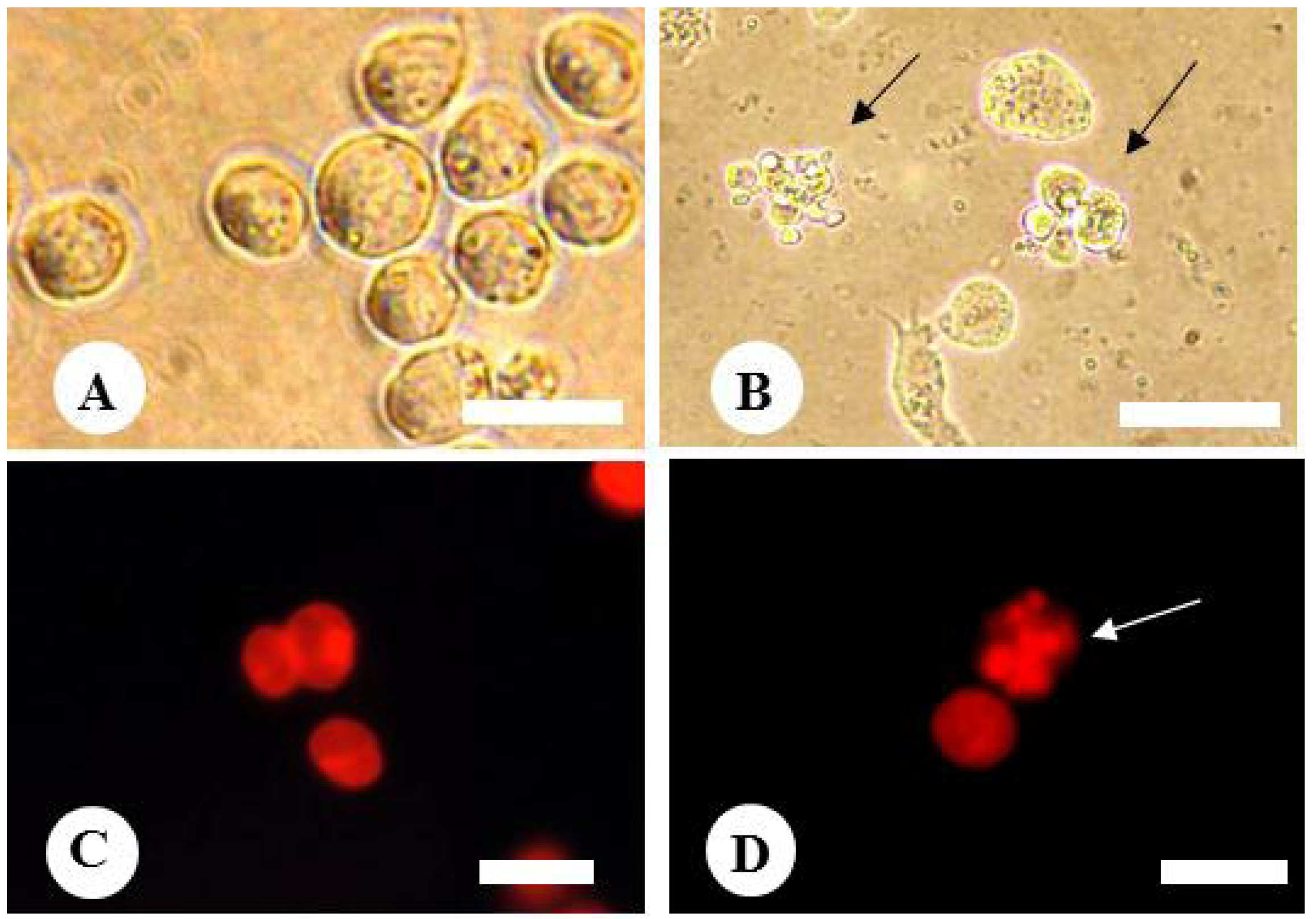
2.5. Effect of Fruit Extracts of Natsuhaze on Apoptosis-Induction of HL-60 Cells

3. Discussion
4. Experimental Section
4.1. Fruits
4.2. Anthocyanins
4.3. Total Polyphenol
4.4. Antioxidant Activity
4.5. Cells and Cell Culture
4.6. Treatment of Cells with Fruit Extracts
4.7. Determination of Nuclear Morphology
4.8. DNA Extraction and Agarose Gel Electrophoresis
5. Conclusions
Acknowledgments
References
- Retamales, J.B.; Hancock, J.F. Blueberries; CABI Publishing: Wallingford, UK, 2012. [Google Scholar]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; García, J.L.; Rosado, J.L.; Goñi, I. The contribution of fruits and vegetables to dietary intake of polyphenols and antioxidant capacity in a Mexican rural diet: Importance of fruit and vegetable variety. Food Res. Int. 2011, 44, 1182–1189. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, H.; Camp, M.J.; Ehlenfeldt, M.K. Flavonoid constituents and their contribution to antioxidant activity in cultivars and hybrids of rabbiteye blueberry (Vaccinium ashei Reade). Food Chem. 2012, 132, 855–864. [Google Scholar] [CrossRef]
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruits and cancer. II. Mechanisms. Cancer Causes Control 1991, 2, 427–442. [Google Scholar] [CrossRef]
- Kraft, T.F.B.; Schmidt, B.M.; Yousef, G.G.; Knight, C.T.G.; Cuendet, M.; Kang, Y.H.; Pezzuto, J.M.; Seigler, D.S.; Lila, M.A. Chemopreventive potential of wild lowbush blueberry fruits in multiple stages of carcinogenesis. J. Food Sci. 2005, 70, 159–166. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Yamazaki, T. Ericaceae. In Wild Flowers of JAPAN, Woody Plants (in Japanese); Satake, Y., Hara, H., Watari, S., Tominari, T., Eds.; Heibonsha: Tokyo, Japan, 1989; Volume 2, pp. 122–156. [Google Scholar]
- Hirai, M.; Yoshimura, S.; Ohsako, T.; Kubo, N. Genetic diversity and phylogenetic relationships of the endangered species Vaccinium sieboldii and Vaccinium ciliatum (Ericaceae). Plant Syst. Evol. 2010, 287, 75–84. [Google Scholar] [CrossRef]
- Kunitake, H.; Tsuda, H.; Takagi, R.; Ohno, Y.; Kuroki, Y.; Yoshioka, K.; Kage, T.; Ito, T.; Komatsu, H. Possibility of wild blueberry shashanbo (Vaccinium bracteatum Thunb.) as a rootstock for cultivation of northern highbush blueberry in warm region (In Japanese with English abstract). Hortic. Res. (Japan) 2006, 5, 105–110. [Google Scholar]
- Lee, J.; Wrolstad, R.E. Extraction of anthocyanins and polyphenolics from blueberry processing waste. J. Food Sci. 2004, 69, 564–573. [Google Scholar]
- Riihinen, K.; Jaakola, L.; Karenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and “northblue” blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Diaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) growing in southern Chile. J. Soil. Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar]
- Wang, S.Y.; Camp, M.J.; Ehlenfeldt, M.K. Antioxidant capacity and α-glucosidase inhibitory activity in peel and flesh of blueberry (Vaccinium spp.) cultivars. Food Chem. 2012, 132, 1759–1768. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Goñi, I. Definition of the Mediterranean diet based on bioactive compounds. Crit. Rev. Food Sci. Nutr. 2009, 49, 145–152. [Google Scholar] [CrossRef]
- Liu, D.; Colina-Ibarra, J.; Kakuda, A.; Hue, S.J. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C and β-carotene mixtures on the DPPH free radical. LWT Food Sci. Technol. 2008, 41, 1344–1349. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Pérez-Jiménez, J.; Goñi, I. Dietary fiber and associated antioxidants in fruit and vegetables. In Fruit and Vegetable Phytochemicals; de La Rosa, L.A., Álvarez-Parilla, E., González-Aguilar, G.A., Eds.; Wiley: Ames, IA, USA, 2010; pp. 223–234. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Blomhoff, R.; Carlsen, M.H.; Andersen, L.F.; Jacobs, D.R. Health benefits of nuts: Potential role of antioxidants. Br. J. Nutr. 2006, 96, 52–60. [Google Scholar] [CrossRef]
- Gosslau, A.; Chen, K.Y. Nutraceuticals, apoptosis, and disease prevention. Nutrition 2004, 20, 95–102. [Google Scholar] [CrossRef]
- Ghahremani-majd, H.; Dashti, F.; Dastan, D.; Mumivand, H.; Hadian, J.; Esna-Ashari, M. Antioxidant and Antimicrobial Activities of Iranian Mooseer (Allium hirtifolium Boiss) Populations. Hortic. Environ. Biotechnol. 2012, 53, 116–122. [Google Scholar] [CrossRef]
- Schroeter, H.; Boyd, C.; Spencer, J.P.E.; Williams, R.J.; Cadenas, E.; Rice-Evans, C. MAPK signaling in neurodegeneration: Influences of flavonoids and of nitric oxide. Neurobiol. Aging 2002, 23, 861–880. [Google Scholar] [CrossRef]
- Prior, R.L.; Joseph, J. Berries and fruits in cancer chemoprevention. In Phytopharmaceuticals in Cancer Chemoprevention; Bagchi, D., Preuss, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 465–479. [Google Scholar]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 132, 855–864. [Google Scholar]
- Koca, I.; Karadeniz, B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea Region of Turkey. Sci. Hortic. 2009, 121, 447–450. [Google Scholar] [CrossRef]
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, H.; Camp, M.J.; Ehlenfeldt, M.K. Flavonoid constituents and their contribution to antioxidant activity in cultivars and hybrids of rabbiteye blueberry (Vaccinium ashei Reade). Food Chem. 2012, 132, 855–864. [Google Scholar] [CrossRef]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of apoptosis in cancer cells by Bilberry (Vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem. 2003, 51, 68–75. [Google Scholar] [CrossRef]
- Kalt, W.; Ryan, D.A.J.; Duy, J.C.; Prior, R.L.; Ehlenfeldt, M.K.; vander Kloet, S.P. Interspecific variation in anthocyanins, phenolics, and antioxidant capacity among genotypes of highbush and lowbush blueberries (Vaccinium section Cyanococcus spp.). J. Agric. Food Chem. 2001, 49, 4761–4767. [Google Scholar]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, P.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar]
- Prior, R.L. Absorption and metabolism of anthocyanins: Potential health effects. In Phytochemicals: Mechanisms of Action; Meskin, M.S., Bidlack, W.R., Davies, A.J., Lewis, D.S., Randolph, R.K., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–16. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; Gonzalez-Paramas, A.M.; Torronen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar]
- Määttä-Riihinen, K.R.; Kahkonen, M.P.; Torronen, A.R.; Heinonen, I.M. Catechins and proanthocyanidins in berries of Vaccinium species and their antioxidant activity. J. Agric. Food Chem. 2005, 53, 8485–8491. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Ho, K.Y.; Tsai, C.C.; Huang, J.S.; Lin, T.C.; Hsu, Y.F.; Lin, C.C. Antioxidant activity of tannin compounds from Vaccinium vitis-idaea L. J. Pharm. Pharmacol. 1999, 51, 1075–1078. [Google Scholar] [CrossRef]
- Nandakumar, V.; Singh, T.; Katiyar, S. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Erdman, J.W., Jr.; Lila, M.A. Differential effects of blueberry proanthocyanidins on androgen sensitive and insensitive human prostate cancer cell lines. Cancer Lett. 2006, 231, 240–246. [Google Scholar] [CrossRef]
- Ballington, J.R.; Ballinger, W.E.; Maness, E.P. Interspecific differences in the percentage of anthocyanins, aglycone-sugars in the fruit of seven species of blueberries. J. Am. Soc. Hortic. Sci. 1987, 112, 859–864. [Google Scholar]
- Singleton, U.L.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-posphotungustic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Suda, I. Determination of DPPH radical scavenging activity by spectrophotometry. In The Methods of Food Functions Analysis (In Japanese); Shinohara, K., Suzuki, T., Kaminogawa, S., Eds.; Korin: Tokyo, Japan, 2000; pp. 218–220. [Google Scholar]
- Yamasaki, M.; Kawabe, A.; Nishimoto, K.; Madhyastha, H.; Sakakibara, Y.; Suiko, M.; Okamoto, T.; Suda, T.; Uehira, K.; Nishiyama, K. Dihydro-alpha-lipoic acid has more potent cytotoxicity than alpha-lipoic acid. In Vitro Cell Dev. Biol. Anim. 2009, 45, 275–280. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Tong, C.B.S. Variability in antioxidant activity in blueberry and correlations among different antioxidant assays. J. Am. Soc. Hortic. Sci. 2002, 127, 238–244. [Google Scholar]
- Connor, A.M.; Luby, J.J.; Tong, C.B.S.; Finn, C.E.; Hancock, J.F. Genotypic and environmental variation in antioxidant activity, total phenolic content, and anthocyanin content among blueberry cultivars. J. Am. Soc. Hortic. Sci. 2002, 127, 89–97. [Google Scholar]
- Rowland, L.J.; Hancock, J.F.; Bassil, N.V. Blueberry. In Genetics, Genomics and Breeding of Berries; Folta, K.M., Kole, C., Eds.; Science Publisher: Enfield, CT, USA, 2011; pp. 1–40. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsuda, H.; Kunitake, H.; Kawasaki-Takaki, R.; Nishiyama, K.; Yamasaki, M.; Komatsu, H.; Yukizaki, C. Antioxidant Activities and Anti-Cancer Cell Proliferation Properties of Natsuhaze (Vaccinium oldhamii Miq.), Shashanbo (V. bracteatum Thunb.) and Blueberry Cultivars. Plants 2013, 2, 57-71. https://doi.org/10.3390/plants2010057
Tsuda H, Kunitake H, Kawasaki-Takaki R, Nishiyama K, Yamasaki M, Komatsu H, Yukizaki C. Antioxidant Activities and Anti-Cancer Cell Proliferation Properties of Natsuhaze (Vaccinium oldhamii Miq.), Shashanbo (V. bracteatum Thunb.) and Blueberry Cultivars. Plants. 2013; 2(1):57-71. https://doi.org/10.3390/plants2010057
Chicago/Turabian StyleTsuda, Hirotoshi, Hisato Kunitake, Ryoko Kawasaki-Takaki, Kazuo Nishiyama, Masao Yamasaki, Haruki Komatsu, and Chizuko Yukizaki. 2013. "Antioxidant Activities and Anti-Cancer Cell Proliferation Properties of Natsuhaze (Vaccinium oldhamii Miq.), Shashanbo (V. bracteatum Thunb.) and Blueberry Cultivars" Plants 2, no. 1: 57-71. https://doi.org/10.3390/plants2010057
APA StyleTsuda, H., Kunitake, H., Kawasaki-Takaki, R., Nishiyama, K., Yamasaki, M., Komatsu, H., & Yukizaki, C. (2013). Antioxidant Activities and Anti-Cancer Cell Proliferation Properties of Natsuhaze (Vaccinium oldhamii Miq.), Shashanbo (V. bracteatum Thunb.) and Blueberry Cultivars. Plants, 2(1), 57-71. https://doi.org/10.3390/plants2010057





