1. Introduction
Left-right (L-R) asymmetry is a basic feature of the vertebrate body plan. It encompasses asymmetric organ placement within the coelom, as well as intrinsic chirality of organs, like the heart or the gut tube [
1,
2]. Any failure of establishing proper organ position and asymmetric morphogenesis along the L-R axis during embryonic development may result in
heterotaxia, which means non-concordant allocation of organ anlagen to the left and right body side. Such a condition, which is also named
situs ambiguus, causes a wide spectrum of congenital heart defects, such as atrial septal defects (ASDs), ventricular septal defects (VSDs), atrioventricular septum defects (AVSD), transposition of the great arteries (TGA), double outlet right ventricle (DORV) and aortic arch (AA) anomalies [
3,
4]. Moreover, cardiac conduction disease is also common in heterotaxy syndrome [
5].
While L-R asymmetry is present in many organisms, it remains a debated issue when and, in particular, how L-R asymmetry is established [
6]. There is agreement in the field that there are three phases involved in L-R axis development [
7]. An early phase exists, during which the L-R-axis is oriented relative to the anteroposterior and dorsoventral axes. The second phase involves translation of this first decision into asymmetric gene expression. In the third step, L-R asymmetry information is transduced into asymmetric morphogenesis.
Surprisingly, the first step in L-R axis determination appears to be the least conserved. Several mechanisms have been found in organisms, such as a asymmetric imprinting of chromatids, chiral cytoskeleton, asymmetric distribution of ion channels and pumps, leading to asymmetric distribution of membrane voltage, asymmetric transport of molecules, such as serotonin through gap junctions, planar cell polarity, asymmetric cell migration and, last, but not least, ciliary flow [
6]. These different mechanisms of establishing the L-R axis are utilized in organisms to a different extent. In higher vertebrates, symmetry breaking occurs during early gastrulation, although the exact timing has not been fully established, due to the difficulties in studying the mammalian embryo before implantation. In mice, it is believed that the nodal flow, a directional fluid flow generated by cilia, which are precisely positioned on the ventroposterior side of nodal cells in the gastrulating mouse embryo, establishes the L-R axis [
1]. An alternative mechanism present in the chicken and possibly in the porcine embryo induces asymmetric cell migration around Hensen’s node, which precedes asymmetric gene expression [
8]. In the chicken, asymmetric distribution of membrane voltage, asymmetric transport of serotonin through gap junctions and the planar cell polarity pathway are upstream of establishing L-R asymmetry at the node [
2].
The most prominent signalling cascade involved in determining L-R asymmetry is the evolutionary conserved
NODAL/
PITX2 pathway, which is induced at the embryonic organizer and determines the left-sided identity of the lateral plate mesoderm (
Figure 1) [
9,
10]. On the right side,
PITX2 is actively repressed by
FGF8 and
SNA1 in the chick embryo [
11,
12]. It has been shown in various animal models, including chicken and
Xenopus, that these signalling cascades are preceded by asymmetric ion flux across the embryo, which may lead to an asymmetric accumulation of low molecular weight determinants, like serotonin [
13].
There is only a limited amount of data on the actual impact of these L-R signalling events on asymmetric organogenesis at later developmental stages. It has been shown, for example, that the asymmetric bending of the gut tube is under the control of
PITX2, which induces left-sided tissue condensation in the dorsal mesentery [
14]. Gut looping in the zebrafish depends on asymmetric migration of lateral plate mesoderm, and NODAL signalling is essential for proper gut looping [
15]. The molecular mechanisms that govern cardiac looping are poorly understood. Some progress has been made in the zebrafish embryo, which apparently uses NODAL and BMP signals to control asymmetric cell migration [
16,
17,
18]. However, whether these pathways are evolutionary conserved and also utilized in higher vertebrates is presently unclear.
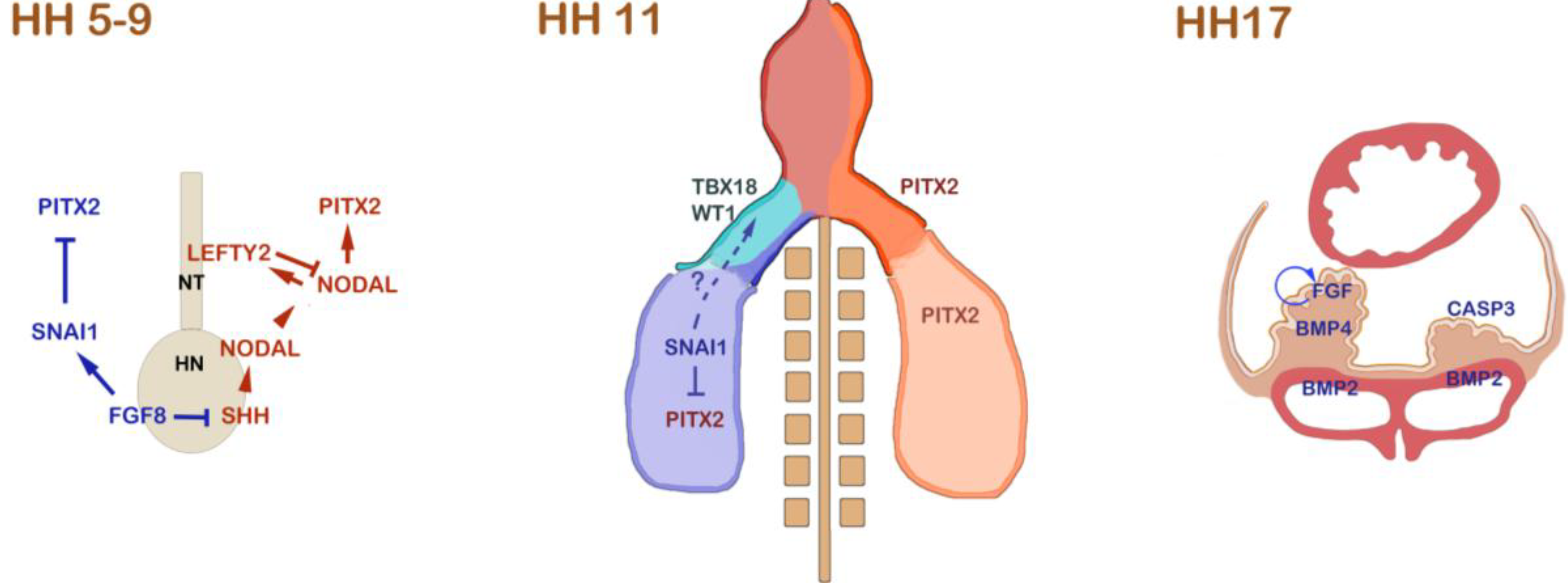
Figure 1.
Left-right asymmetric signalling events that are involved in proepicardium (PE) formation in the chick. During gastrulation and the onset of neurulation from HH stage 5 to 9, the
NODAL/
PITX2 pathway is induced by asymmetric SHH signalling at Hensen’s node (HN). SHH induces
NODAL at the left side of the node. NODAL induces itself in the left lateral plate mesoderm, where it induces
PITX2. In order to prevent Nodal from spreading to the right side, NODAL induces a feedback inhibitor,
LEFTY2, in the developing notochord (NT). On the right side of the node, FGF8 antagonizes
SHH and induces
SNAI1 in the right lateral plate mesoderm. SNAI1 acts as a transcriptional repressor of
PITX2 on the right side. At HH stage 11, the embryonic heart is depicted. On the left side of the embryo, including the left inflow tract,
PITX2 is expressed. In the right lateral plate mesoderm,
SNAI1 still lingers and is subsequently downregulated. At this stage, the first PE marker gene expression can be detected.
TBX18 is initially expressed on the right side, and the right-sided expression of
WT1 follows shortly thereafter [
23]. It has been shown that manipulation of FGF8/SNAI1 interferes with asymmetric expression of
TBX18 and
WT1 [
23]. The mechanism of how SNAI1 might regulate asymmetric PE marker gene expression remains unclear; however, it has been suggested that SNAI1 might be responsible for asymmetric mobilization of PE progenitor cells in the lateral plate. At later stages, up to HH stage 17, BMP and FGF signalling have been shown to be responsible for the specification and the asymmetric PE outgrowth. BMP is required at low concentration to maintain PE marker gene expression. This is realized by a differential expression of
BMP4 at low levels in the PE and of
BMP2 at high levels in the
sinus venosus. A feedback loop of FGF signalling is crucial for the survival and growth of PE cells on the right side [
24]. The lack of FGF on the left side leads to massive retardation of the PE primordium and upregulation of
CASP3, which induces apoptosis
Figure 1.
Left-right asymmetric signalling events that are involved in proepicardium (PE) formation in the chick. During gastrulation and the onset of neurulation from HH stage 5 to 9, the
NODAL/
PITX2 pathway is induced by asymmetric SHH signalling at Hensen’s node (HN). SHH induces
NODAL at the left side of the node. NODAL induces itself in the left lateral plate mesoderm, where it induces
PITX2. In order to prevent Nodal from spreading to the right side, NODAL induces a feedback inhibitor,
LEFTY2, in the developing notochord (NT). On the right side of the node, FGF8 antagonizes
SHH and induces
SNAI1 in the right lateral plate mesoderm. SNAI1 acts as a transcriptional repressor of
PITX2 on the right side. At HH stage 11, the embryonic heart is depicted. On the left side of the embryo, including the left inflow tract,
PITX2 is expressed. In the right lateral plate mesoderm,
SNAI1 still lingers and is subsequently downregulated. At this stage, the first PE marker gene expression can be detected.
TBX18 is initially expressed on the right side, and the right-sided expression of
WT1 follows shortly thereafter [
23]. It has been shown that manipulation of FGF8/SNAI1 interferes with asymmetric expression of
TBX18 and
WT1 [
23]. The mechanism of how SNAI1 might regulate asymmetric PE marker gene expression remains unclear; however, it has been suggested that SNAI1 might be responsible for asymmetric mobilization of PE progenitor cells in the lateral plate. At later stages, up to HH stage 17, BMP and FGF signalling have been shown to be responsible for the specification and the asymmetric PE outgrowth. BMP is required at low concentration to maintain PE marker gene expression. This is realized by a differential expression of
BMP4 at low levels in the PE and of
BMP2 at high levels in the
sinus venosus. A feedback loop of FGF signalling is crucial for the survival and growth of PE cells on the right side [
24]. The lack of FGF on the left side leads to massive retardation of the PE primordium and upregulation of
CASP3, which induces apoptosis
![Jdb 01 00126 g001]()
In this review, we focus on asymmetric development of the proepicardium (PE), which is an easily detectable morphological asymmetry of the embryonic heart, which is present in many vertebrate species. The PE is a cluster of progenitor cells that forms on the cardiac inflow tract. Its purpose is the accumulation and, subsequently, the transfer of progenitor cells to the embryonic heart [
19]. This is accomplished via the process of epicardialization of the myocardial surface in which PE cells attach to heart and cover it with an epicardium [
20]. The PE and the epicardium harbour several subpopulations of cells, which give rise to various cell types of the coronary blood vessel system, including smooth muscle cells, fibroblasts and endothelial cells [
21] (
Figure 2). The formation of the epicardium is a vital process, not only for the formation of coronary blood vessels, but also for the growth and maturation of the myocardial wall [
22]. In order to ensure proper PE formation, several signalling factors, like BMP and FGF, which initially have been identified in the chick, are important for cell specification and drive proepicardial growth and survival [
23,
24,
25,
26]. In addition, PE formation in the chicken embryo has been shown to be a target of right-sided signalling during early gastrulation involving
FGF8/
SNAI1, which promotes its asymmetric establishment on the cardiac inflow tract [
27]. Here, we will discuss the available knowledge on asymmetric PE development among vertebrates and put it into the context of other known inflow tract asymmetries, including the formation of the cardiac pacemaker.
Figure 2.
The PE is a heterogeneous cell cluster at the venous pole. The PE develops on the right
sinus venosus, which is a transient region, and the connection between the vitelline veins and the atrium of the embryonic heart. At the venous pole, cells are added constantly to the heart tube. Some of these cells are also recruited into the PE. The PE consists of an outer layer of mesothelial cells, which form the characteristic protrusions that project into the pericardial cavity. The core of the PE harbours matrix-producing mesenchymal cells from the
sinus venosus. Furthermore, endothelial progenitor cells are being recruited into the PE cluster, either from the sinus endothelium, the liver primordium or both. This leads to a complex mixture of vasculogenic and fibroblast progenitor cells in the PE that are ultimately transferred to the heart (modified from [
23]).
Figure 2.
The PE is a heterogeneous cell cluster at the venous pole. The PE develops on the right
sinus venosus, which is a transient region, and the connection between the vitelline veins and the atrium of the embryonic heart. At the venous pole, cells are added constantly to the heart tube. Some of these cells are also recruited into the PE. The PE consists of an outer layer of mesothelial cells, which form the characteristic protrusions that project into the pericardial cavity. The core of the PE harbours matrix-producing mesenchymal cells from the
sinus venosus. Furthermore, endothelial progenitor cells are being recruited into the PE cluster, either from the sinus endothelium, the liver primordium or both. This leads to a complex mixture of vasculogenic and fibroblast progenitor cells in the PE that are ultimately transferred to the heart (modified from [
23]).
2. Asymmetric PE Morphology among Vertebrates
Asymmetric PE development can be observed in various vertebrate model organisms (
Figure 3); however, to our knowledge, PE development has not yet been studied in the human heart. In many cases PE formation is strongly biased to the right side [
28]. In the chick, the first morphological indication of PE development can be observed at the HH stage [
14]. On the right side of the cardiac inflow tract, or
sinus venosus, initial villous protrusions mark the morphological onset of PE formation [
29]. This asymmetric morphology is preceded by the expression of conserved marker genes, like
TBX18,
WT1 and
TCF21, which start to be asymmetrically expressed in the right sinus at HH stage 12 [
23].
The PE protrusions become more pronounced by enhanced proliferation, until the PE has reached its full size at HH stage 17, which is shortly before its attachment to the myocardium [
24]. The PE at this stage consists of an outer layer of cuboidal mesothelial cells and an inner core of mesenchymal cells, which produces a significant amount of extracellular matrix. In addition, endothelial progenitors are present in the PE, most likely recruited from the underlying sinus of the vitelline veins, although other sources, like liver sinusoids, have also been suggested as putative site of origin [
30,
31,
32]. While the right PE will subsequently attach to the dorsal side of the ventricle and colonizes the heart, the left-sided anlage displays a strongly retarded development and will not contribute any cells to the heart [
21]. In the chick, the PE-derived cells are transferred to the heart via a right-sided tissue bridge that is established at HH stage 18 [
21]. Since, by that stage, the PE has reached a significant size on the right inflow tract, the outer mesothelial cells eventually come in contact with the perpetually contracting ventricle. The attachment of the mesothelial villi leads to the release of matrix from the core of the PE, which facilitates the formation of the tissue bridge, along which PE cells are continuously migrating over the myocardial surface [
23].
In contrast to the chick, the mouse PE does not display a prominent left-right asymmetry. The original two PE anlagen on the venous pole apparently fuse to form one medial cluster [
33]. Furthermore, the transfer of cells differs from the chick. Rather than forming a tissue bridge, the highly adhesive villous protrusions are plucked off by the beating heart, resulting in the formation of a patchwork of epicardial islands, which subsequently become confluent to form a continuous epicardial layer [
34]. In addition to that, the release of free-floating cell cysts from the PE, which cross the pericardial cavity and passively attach to the heart, have been described [
35].
In
Xenopus, like in the chick, the PE forms on the right side of the
sinus venosus [
36,
37]. At stage 41, the PE is visible as a cone-shaped cluster of
TBX18-positive mesothelial cells. The time window of PE formation is much shorter than in the chick, and the PE in
Xenopus embryos can be observed only for a couple of hours at stage 41, before it makes contact with the heart. The cellular transfer is accomplished via a tissue bridge, which persists up to stage 46. PE development in
Xenopus displays the strongest bias of all animal models, since no morphological or molecular evidence for a left-sided PE anlage has been obtained. This might be due to the accelerated PE development in
Xenopus, but also, it could be the result of the very early establishment of the left-right axis during the first cell division in this model organism. In fact, right-sided PE formation appears to be common among amphibians, since it can also be observed in the
axolotl [
38].
In various fish species, PE development appears to be less stringent in terms of bilateral asymmetry. In zebrafish, the first signs of the PE can be found after two days post-fertilization on the
sinus venosus [
39,
40]. However, the spatial relation of the PE to the cardiac inflow tract and the surrounding pericardial cavity are still under investigation. Nonetheless, it has been suggested that PE cells are released into the pericardial cavity, rather than being transferred via a tissue bridge. The mechanisms by which free-floating cells or epithelial cysts are delivered to the heart remain to be investigated. The analysis of PE marker genes indicates the existence of two PE anlagen at the venous pole, but the potential contribution of both primordia to the epicardium is presently unknown [
39]. The question of the bilateral origin of the PE was experimentally tested by inducing
cardia bifida through knockdown of miles apart (
mil) or casanova (
cas) genes. In contrast to the chick, where in
cardia bifida embryos, only the right heart develops a PE [
29], in the zebrafish embryo, both heart tubes develop WT1-positive PE cells [
39]. These data suggest differences between chick and zebrafish with regard to asymmetric PE development.
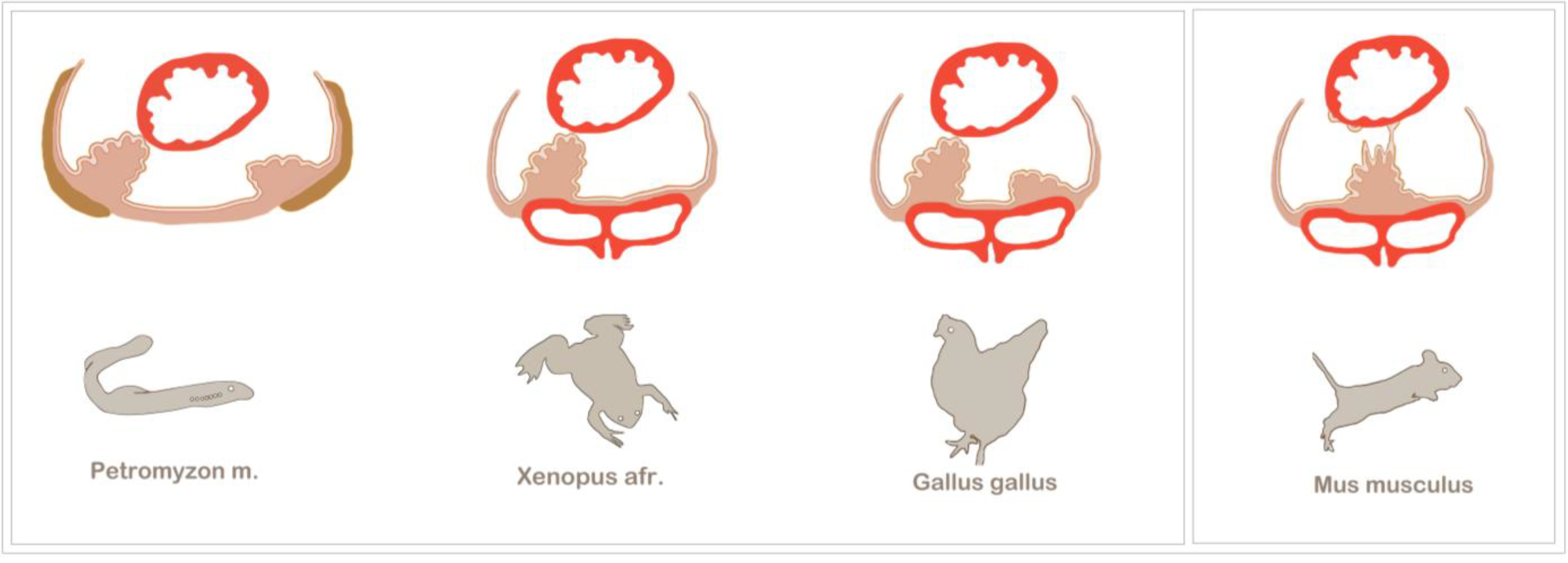
Figure 3.
Examples of asymmetric and symmetric PE development among vertebrates. The PE generally forms at the venous pole of the heart and is in close relation to the major veins. Asymmetric PE formation is a conserved feature of vertebrate development. Among the most prominent model organisms are the chick and
Xenopus, which both exhibit a right-sided PE cluster that establishes a tissue bridge to the heart. The most basic vertebrate studied so far is the lamprey,
Petromyzon marinus, which also displays a right-sided tissue bridge to the heart. Additionally, in the lamprey, the PE is in close spatial relation to the pronephros (olive), and after epicardium formation, the PE cluster gives rise to the highly vascularized pronephric glomeruli. In mouse embryos, asymmetric PE formation has not been observed; instead, the bilaterally formed PE clusters fuse at the midline of the
sinus venosus, and PE cell transfer is either accomplished by the release of PE cysts into the pericardial cavity or by being plucked off by the contracting ventricle (modified from [
23]).
Figure 3.
Examples of asymmetric and symmetric PE development among vertebrates. The PE generally forms at the venous pole of the heart and is in close relation to the major veins. Asymmetric PE formation is a conserved feature of vertebrate development. Among the most prominent model organisms are the chick and
Xenopus, which both exhibit a right-sided PE cluster that establishes a tissue bridge to the heart. The most basic vertebrate studied so far is the lamprey,
Petromyzon marinus, which also displays a right-sided tissue bridge to the heart. Additionally, in the lamprey, the PE is in close spatial relation to the pronephros (olive), and after epicardium formation, the PE cluster gives rise to the highly vascularized pronephric glomeruli. In mouse embryos, asymmetric PE formation has not been observed; instead, the bilaterally formed PE clusters fuse at the midline of the
sinus venosus, and PE cell transfer is either accomplished by the release of PE cysts into the pericardial cavity or by being plucked off by the contracting ventricle (modified from [
23]).
![Jdb 01 00126 g003]()
The sturgeon is a more archaic fish species in which epicardium formation has been thoroughly analysed [
41]. After four days post-hatching, two bilateral PE anlagens can be observed, which subsequently fuse. Although there is seemingly no evidence for an asymmetric PE induction, the sturgeon makes use of tissue bridges, which have been found to be associated with asymmetric PE development in higher vertebrates. However, tissue bridges are not the only mode of cell transfer to the heart in the sturgeon. Although the conus myocardium is covered with a continuous epicardial layer, it has been suggested that the ventricle is colonized by PE cells that have been released into the pericardial cavity. The tissue bridges are not transient, like in the chick or
Xenopus, but persist as a connection between the coronary vascular plexus and the
sinus venosus and, also, carry cardiac nerves [
41].
From an evolutionary point of view, the lamprey,
Petromyzon marinus, is the most distant and primitive of all vertebrate model organisms that have been used to study PE development [
42]. The lamprey is a jawless parasitic fish, which, together with the hagfish, belongs to the group of agnathans. The lamprey has a peculiar anatomy, which brings the PE in close evolutionary relation to the pronephros. Evidence for such a relation can be found in higher vertebrates at the molecular level. For example, in the chick, the PE and the intermediate mesoderm, which gives rise to the nephric system, share the expression of several transcriptions factors, like
TBX18,
WT1 and
TCF21 [
23,
43]. Moreover, the membrane protein, podoplanin, is expressed in podocytes of the kidney, as well as in the PE and the coelomic epithelium [
44]. For further discussion on this subject, see the article by Cano
et al. in this volume [
45]. The lamprey initially develops two PE primordia, but similar to other organisms, only the right-sided PE cluster forms a tissue bridge and contributes to the epicardium. After epicardium formation, the PE clusters develop into the highly vascularized pronephric external glomeruli, underlining the vasculogenic potential as a conserved characteristic feature of the PE [
42].
3. The Generation of Left-Right Asymmetry
The chick has served as one of the main models for the investigation of PE development, especially for the analysis of the molecular mechanisms underlying asymmetric PE formation. In order to clarify the molecules and factors involved in generating this biased development, we will briefly introduce the main signalling pathways that establish left-right asymmetry and, thereby, mainly focus on the chick embryo.
The core-signalling pathway in establishing left identity in the mesoderm is the highly conserved
NODAL/
PITX2 signalling cascade (
Figure 1) [
9,
22]. This pathway is induced at the embryonic organizer or Hensen’s node during gastrulation. Asymmetric, leftward movement of cells around the node [
8] causes a displacement of cells expressing
SHH and
FGF8 and, thereby, establishing their asymmetric expression patterns on the left and right side of Hensen’s node, respectively. SHH induces the TGFß molecule,
NODAL, which is highly diffusible and spreads into the lateral plate mesoderm, where it induces transcription factors, like
PITX2 and
BAPX1, which ultimately determine left identity [
2].
There are several mechanisms that ensure that only one side acquires left-sided identity. The first step is the inhibition of
SHH on the right side of the node by FGF8, which basically inhibits the induction of the
NODAL/
PITX2 cascade on the right side [
11]. Furthermore, FGF8 induces the transcriptional repressor,
SNA1, in the right lateral plate mesoderm, which represses
PITX2 on the right side. Since NODAL is a diffusible morphogen, it is paramount to prevent it from spreading to the right side of the embryo. This is accomplished by NODAL itself, which induces another TGFß molecule,
LEFTY2 [
46].
LEFTY2 is expressed in the embryonic midline, has a higher diffusion rate than NODAL and is able to inhibit signalling by competing for the NODAL receptor complex. This biochemical activity establishes the midline barrier, which limits diffusion of NODAL towards the right side [
47]. There are many more molecules and mechanisms involved in the establishment of the
NODAL/
PITX2 pathway on the left side of the embryo, but these cannot be discussed into further depth in the interest of brevity; the reader is referred elsewhere for further information [
48].
Instead, we also want to mention earlier mechanisms that have been suggested to break initial embryonic symmetry. In chick and frog embryos, an asymmetric ion flux generated by H-/V-ATPases has been reported, which occurs before the onset of the
NODAL/
PITX2 expression and supposedly drives the asymmetric accumulation of low molecular weight determinants, like serotonin [
13]. In addition, the presence of apoptotic cells, which reside in the primitive streak, might act as an early midline barrier (before LEFTY2 and the notochord comes into play) that prevents asymmetrically distributed small molecules from leaking to the other side [
49]. However, it is still not fully understood how these events feed into the asymmetric expression of
SHH and
NODAL, although, in the chick embryo, evidence has been obtained for a control of asymmetric cell migration by asymmetric ion flux [
8].
Another interesting finding is a highly conserved role for tubulin, which has been proposed to be responsible for asymmetric distribution of maternal factors during the first cell division in
Xenopus [
50]. Likewise, tubulin is involved in the chirality of
Arabidopsis and asymmetric organogenesis in
Caenorhabditis elegans. These observations largely contributed to the idea that asymmetric rearrangements of the cytoskeleton are the driving force of embryonic left-right asymmetry. Furthermore, it has been emphasized that the cellular mechanisms behind left-right axis determination, in fact, show resemblance with the planar cell polarity (PCP) pathway [
51].
4. Molecular Control of Asymmetric PE Development
Since the chick has been amongst the best-studied organisms for both PE development, as well as left-right asymmetry, it has taken a leading role as a model for the investigation of right-sided PE development. The experimental induction of
cardia bifida in chick embryos demonstrated that although the embryos develop two independently beating heart tubes, only the right inflow tract has a PE, and consequently, only the right heart became covered with an epicardium [
33]. Consequently, the left heart tube is devoid of an epicardium, which has a negative impact on the maturation of the myocardial wall. These experiments clearly establish that the PE has a right-sided identity and that the formation of the heart is not necessary for the induction of the PE. Nonetheless, it is tempting to propose a potential coordination of sidedness of PE formation and the direction of heart looping, which is also strictly rightward.
The initial hypothesis for the molecular control of PE development included the idea that PITX2 might act as a PE repressor on the left side, since
PITX2 is strongly expressed in the left cardiac inflow tract. However, gain-of-function experiments show that the
NODAL/
PITX2 pathway does not interfere with PE formation [
27]. Significantly, the forced expression of
SHH on the right side produces aberrant cardiac looping, but, at the same time, is unable to interfere with right-sided PE marker gene expression. These data provide further evidence for an independent regulation of these two events, which can be experimentally uncoupled. Even the overexpression of
PITX2 in mesodermal progenitor cells does not change the asymmetric PE transcriptional program in the cardiac inflow tract [
27]. The gain and loss of the right-sided
FGF8/
SNAI1 pathway, however, does strongly affect right-sided PE marker gene expression. Ectopic
FGF8 or
SNAI1 is able to induce
TBX18 and
WT1 in the left sinus, and likewise, the loss of
FGF8/
SNAI1 leads to a loss of these markers. These observations have led to the concept that right-sided
FGF8 and
SNAI1 are, in fact, more than repressive factors, but that they also have inductive capacity and are directly involved in asymmetric morphogenesis of the inflow tract [
27]. However, the mechanisms of how these factors actually induce right-sided PE formation are still under investigation.
FGF8 is expressed at gastrula stages, and
SNAI1 is maintained in the lateral plate mesoderm until the onset of heart tube formation at stage HH10. This makes it necessary to identify potential downstream targets of
SNAI1 that might interfere with PE development.
SNAI1 is known to be involved in epithelial-mesenchymal transition (EMT) and promotion of cell invasion [
52]. Therefore, one might hypothesize that
SNAI1 is enhancing the invasion of PE progenitor cells from the lateral plate mesoderm, which would ultimately lead to an initial bias by a higher level of PE progenitor cells in the right cardiac inflow tract.
There are several other genes displaying asymmetric expression in the cardiac inflow tract, which have been shown to have an impact on PE formation.
BMP4 starts to display a weak asymmetric expression in the right vitelline vein at HH stage 12 and can also be found in the PE at later stages [
23]. This is particularly interesting, since
BMP2 shows a strong symmetric expression at the venous pole, but not in the PE. It has been demonstrated that there is a dosage-dependent function for BMP, which is crucial for the expression of PE marker genes, like
TBX18 and
WT1 [
23]. A low dosage of BMP is necessary for the maintenance of PE markers, whereas higher concentrations drive a subset of PE cells into the myocardial lineage, which has been confirmed by
in vitro experiments [
23]. Notch signalling has been identified to be responsible for suppressing BMP in the PE in order to limit myocardialization of the cell cluster [
53].
Another important group of factors that are asymmetrically expressed in the cardiac inflow tract are FGFs. Several FGF ligands and receptors are expressed in the PE and the right
sinus venosus [
24]. The most prominent are
FGF2, which is expressed in the entire PE, and
FGF10 and
FGF12, which are enriched in the villous protrusions. The receptors,
FGFR1,
2 and
4, are particularly expressed in the outer villi of the PE, as well.
In vitro experiments and embryonic manipulations confirmed that these FGFs are crucial for proliferation and survival of the PE cluster [
24]. The inhibition of FGF signalling on the right side causes a drastic increase in the number of apoptotic cells in the right sinus. This leads to a smaller and misshaped PE, which basically resembles the fate of the PE anlage on the left side. The left sinus is strongly apoptotic in wild-type embryos, which can be explained by the absence or vastly reduced levels of FGF signalling. Given the expression patterns of ligands and receptors in the PE, an FGF auto-regulatory loop has been suggested. This would mean that the PE is self-stimulating its own survival on the right sinus to ensure proper villous outgrowth.
By now, a significant amount of data on asymmetric PE development has been accumulated; however, further investigations are necessary to provide a better understanding of how early left-right signalling impacts asymmetric PE formation at the venous pole of the heart.
5. Additional Examples of Asymmetric Inflow Tract Development
The heart is a highly asymmetric organ, right from the initial looping of the heart tube up to the formation of atrial and ventricular chambers at later developmental stages. The arterial and the venous pole of the heart are both undergoing massive asymmetric rearrangements during development. We would like to focus on some examples of asymmetric inflow tract development, which also might be relevant for PE development.
We have already discussed the hypothesis that there might be an asymmetric contribution of PE progenitor cells to the inflow tract under the control of the right-sided
FGF8/
SNAI1 pathway in the chick. It has recently been reported that there is an asymmetric contribution of cells from the posterior second heart field to both poles of the murine heart [
54]. This is particularly interesting, since it has been proposed for the mouse embryo that there is a cranio-caudal patterning of the
Isl1-expressing second heart field by
Fgf8,
Fgf10,
Tbx1 and
Mef2c, which label the anterior part [
55,
56]. The most caudal portion of the second heart field, however, gives rise to the
sinus venosus and, most likely, also, harbours PE progenitor cells [
54]. This is also in agreement with the detection of
Isl1-Cre labelled cells in the murine PE [
57]. Since
Isl1 has a rather broad expression domain in the lateral plate mesoderm, additional investigation is necessary to fully understand the potential contribution of the secondary heart field to the PE.
Pitx2 has been identified as an important determinant of asymmetric inflow tract morphogenesis. Since
Pitx2 is expressed in the left atrium and the left venous system, the loss of
Pitx2 has multiple severe impacts on these tissues. These include atrial isomerism and deformation of the atrio-ventricular valve in
Pitx2 mutants in mice [
58]. In addition, deficiencies of pulmonary myocardium formation have been observed, likely due to a proliferative defect in the pulmonary mesenchyme [
59].
Pitx2 is also responsible for the lateralization of the cardiac pacemaker, which develops at the venous pole [
60]. At developmental stages before the
sinus venosus becomes lateralized, the entire caudal region of the tubular heart displays pacemaker activity. Initially, the dominant pacemaker is on the left side [
61]. Pacemaker activity gradually becomes confined to the sinoatrial node (SAN) on the right side, with the progressive maturation of the
sinus venosus myocardium. During this process,
Pitx2 is required for the establishment of left identity of the left sinus and the future left atrium, thereby limiting the pacemaking capacity to the future SAN on the right side. Pitx2 is able to bind to
Shox2, a master regulator of SAN development, thereby directly inhibiting a SAN–specific transcriptional program in the left
sinus. This asymmetric inhibition has been shown to be crucial to ensure proper pacing of the heart and to prevent atrial fibrillation, a highly prevalent type of cardiac arrhythmia [
62].
It has recently been shown that the progenitor cells of the cardiac pacemaker in the chick are localized in a region posterior to the heart forming fields and that these cells are very early specified by canonical WNT signalling [
63]. This is in close proximity to the presumptive origin of
sinus venosus progenitor cells in the posterior region of the heart field [
64]. It would be interesting to investigate whether the fate of
sinus venosus cells that contribute to the PE is likewise under the control of WNT signals at the early gastrula stage. Although both PE and pacemaker cells are crucial constituents of the right cardiac inflow tract, their asymmetric development is controlled by different, even antagonistic signalling pathways. Ultimately, a master regulator, like
PITX2, has not been identified yet for the right side. The impact of the rather early acting
FGF8/
SNAI1 pathway and the lack of responsiveness to
PITX2 suggest a more complex scenario for the asymmetric development of the PE than mere transcriptional repression. As already outlined, this includes asymmetric specification and survival by BMP and FGF, as well as the postulated invasion of PE progenitor cells into the right
sinus. However, this is not unexpected, since the PE is a heterogeneous cell cluster and composed of cells with potentially diverse origins, which have yet to be fully defined. The PE has a very distinct transcriptional program, including factors like
TBX18,
WT1,
TCF21 and
CFC [
23]. This transcriptional program is shared to some extent with the coelomic pericardial mesoderm, as well as the nephrogenic intermediate mesoderm, both tissues that lie outside the heart forming fields. Therefore, the investigation of the heterogeneity and the origin of the PE might hold the key to understand the diverse differentiation potential of this cell cluster and underline its significance as a highly conserved extra-cardiac cell population accumulating at the cardiac inflow tract.
L-R asymmetry of the PE is found in a number vertebrate species. However, until now, the underlying molecular pathway has only been investigated in the chick embryo. An important question in the future will be whether the pathway of asymmetric PE development is conserved in other species. In this regard the
Xenopus embryo will be a very valuable model organism for such a comparative analysis. In this species, the L-R axis has already been laid down together with the first cell division, and PE development is asymmetric, as in the chick embryo. Will
SNAI1 in
Xenopus be upstream of PE development? Is PE development in this species independent of
Pitx2, as has been shown in the chick embryo? Likewise, the mouse needs to be studied further.
SNAI1 is also in the mouse embryo upstream of L-R asymmetry [
59]. In the
SNAI1 knockout mutant, both heart looping and embryo turning are severely abnormal. PE development in the mouse is clearly symmetric [
29]; however, this might be a secondarily acquired pattern. There is the possibility of an underlying asymmetry, which secondarily is suppressed by some other mechanism. Such a phenomenon is, for example, seen in the case of somite development, which is inherently asymmetric, but asymmetry is suppressed by retinoic acid [
60,
61]. Another important area for future research is to identify the mechanism through which
SNAI1 has an impact on PE development. It is possible that
SNAI1 will induce some other transcription factor, since expression is not maintained until PE-specific gene expression commences. Another possibility is, of course, that downstream of
SNAI1 is a paracrine factor, such as BMP4 [
19]. Single cell resolution can probably only be accomplished in the zebrafish, and therefore, it will be important for defining proepicardial cell lineages to generate transgenic GFP lines in the zebrafish that specifically label the PE [
62,
63]. Such lines will ultimately be needed to define the origin of different PE populations and how asymmetric or symmetric PE formation is accomplished.