-
 Evolution of the Jawed Vertebrate (Gnathostomata) Stomach Through Gene Repertoire Loss: Findings from Agastric Species
Evolution of the Jawed Vertebrate (Gnathostomata) Stomach Through Gene Repertoire Loss: Findings from Agastric Species -
 The Congenital Malformation of the Interatrial Septum—A Review of Its Development and Embryology with Clinical Implications
The Congenital Malformation of the Interatrial Septum—A Review of Its Development and Embryology with Clinical Implications -
 Is Hydra Axis Definition a Fluctuation-Based Process Picking Up External Cues?
Is Hydra Axis Definition a Fluctuation-Based Process Picking Up External Cues?
Journal Description
Journal of Developmental Biology
Journal of Developmental Biology
is an international, peer-reviewed, open access journal on the development of multicellular organisms at the molecule, cell, tissue, organ and whole organism levels published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), PubMed, PMC, PubAg, CAPlus / SciFinder, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 27.1 days after submission; acceptance to publication is undertaken in 5.6 days (median values for papers published in this journal in the second half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Testimonials: See what our editors and authors say about Journal of Developmental Biology.
Impact Factor:
2.5 (2024);
5-Year Impact Factor:
2.8 (2024)
Latest Articles
Influence of Obstructive Uropathy on Cyst Formation and Nephrogenesis: Insights from a Fetal Lamb Model
J. Dev. Biol. 2026, 14(1), 5; https://doi.org/10.3390/jdb14010005 - 9 Jan 2026
Abstract
Obstructive uropathy (OU) during fetal development induces a fetal cystic dysplastic kidney. The mechanisms of cyst formation and the onset of renal dysfunction remain unclear. Determining whether nephrogenic potential persists during fetal life may suggest whether early intervention could preserve renal development. We
[...] Read more.
Obstructive uropathy (OU) during fetal development induces a fetal cystic dysplastic kidney. The mechanisms of cyst formation and the onset of renal dysfunction remain unclear. Determining whether nephrogenic potential persists during fetal life may suggest whether early intervention could preserve renal development. We aimed to evaluate residual nephrogenic activity in fetal cystic dysplastic kidneys using β-catenin and CD10 immunostaining, and to assess whether the site of obstruction influences cystogenesis. After appropriate approval, 20 timed-gestation fetal lambs had OU created at 60 days. Males underwent urethral and urachal ligation (n = 8, 3 lost), and females underwent unilateral ureteric ligation (n = 8, 1 lost). Fetuses were sacrificed at 80 days (n = 6) and 140 days (term, n = 10), comparing kidneys with normal controls of the same gestational age using immunohistochemical staining for β-catenin and CD10. Developing fetal cystic dysplastic kidneys were identified at 80 days. β-catenin staining showed the absence of granular cytoplasmic expression in cystic regions, indicating arrested nephrogenesis. In male models, cysts originated exclusively from proximal tubules. Female models exhibited mixed proximal and distal tubular involvement. CD10 staining confirmed the loss of proximal tubular markers. Renal development remained arrested at term. Cyst formation disrupts renal development early in gestation, which persists until term. Differences in cystogenesis between the models suggest that the site of obstruction influences pathogenic mechanisms.
Full article
(This article belongs to the Special Issue Developmental Biology of the Kidney: From Molecular Mechanisms to Congenital Disorders)
►
Show Figures
Open AccessArticle
Discovery of New Markers for Haemogenic Endothelium and Haematopoietic Progenitors in the Mouse Yolk Sac
by
Guillermo Diez-Pinel, Alessandro Muratore, Christiana Ruhrberg and Giovanni Canu
J. Dev. Biol. 2026, 14(1), 4; https://doi.org/10.3390/jdb14010004 - 6 Jan 2026
Abstract
►▼
Show Figures
Erythro-myeloid progenitors (EMPs) originate from the haemogenic endothelium in the yolk sac via an endothelial-to-haematopoietic transition (EHT) to generate blood and immune cells that support embryo development. Yet, the transitory nature of EHT and the limited availability of molecular markers have constrained our
[...] Read more.
Erythro-myeloid progenitors (EMPs) originate from the haemogenic endothelium in the yolk sac via an endothelial-to-haematopoietic transition (EHT) to generate blood and immune cells that support embryo development. Yet, the transitory nature of EHT and the limited availability of molecular markers have constrained our understanding of the origin, identity, and differentiation dynamics of EMPs. Here, we have refined the annotation of yolk sac haemato-vascular populations in publicly available single-cell RNA sequencing (scRNAseq) datasets from mouse embryos to identify novel molecular markers of haemogenic endothelium and EMPs. By sub-clustering key cell populations followed by pseudotime analysis, we refined cluster annotations and then reconstructed differentiation trajectories. Subsequent differential gene expression analysis between clusters identified novel cell surface markers for haemogenic endothelial cells (Fxyd5 and Scarf1) and EMPs (Fcer1g, Tyrobp, and Mctp1). Further, we have identified candidate signalling and metabolic pathways that may regulate yolk sac haematopoietic emergence and differentiation. The specificity of FXYD5, SCARF1, and FCER1G for haemogenic endothelium and EMPs was validated by immunostaining of the mouse yolk sac. These insights into the transcriptional dynamics in the yolk sac should support future investigation of EHT and haematopoietic differentiation during early mammalian development.
Full article

Figure 1
Open AccessReview
The Interplay of One-Carbon Metabolism, Mitochondrial Function, and Developmental Programming in Ruminant Livestock
by
Kazi Sarjana Safain, Kendall C. Swanson and Joel S. Caton
J. Dev. Biol. 2026, 14(1), 3; https://doi.org/10.3390/jdb14010003 - 3 Jan 2026
Abstract
Maternal nutrition during gestation profoundly influences fetal growth, organogenesis, and long-term offspring performance through developmental programming. Among the molecular mechanisms responsive to maternal nutrient availability, one-carbon metabolism plays a central role by integrating folate, methionine, choline, and vitamin B12 pathways that regulate
[...] Read more.
Maternal nutrition during gestation profoundly influences fetal growth, organogenesis, and long-term offspring performance through developmental programming. Among the molecular mechanisms responsive to maternal nutrient availability, one-carbon metabolism plays a central role by integrating folate, methionine, choline, and vitamin B12 pathways that regulate methylation, nucleotide synthesis, and antioxidant defense. These processes link maternal nutritional status to epigenetic remodeling, cellular proliferation, and redox balance during fetal development. Mitochondria act as nutrient sensors that translate maternal metabolic cues into bioenergetic and oxidative signals, shaping tissue differentiation and metabolic flexibility. Variations in maternal diet have been associated with shifts in fetal amino acid, lipid, and energy metabolism, suggesting adaptive responses to constrained intrauterine environments. This review focuses on the molecular interplay between one-carbon metabolism, mitochondrial function, and metabolomic adaptation in developmental programming of ruminant livestock. Understanding these mechanisms offers opportunities to design precision nutritional strategies that enhance fetal growth, offspring productivity, and long-term resilience in livestock production systems.
Full article
(This article belongs to the Special Issue Mitochondrial Function and Dysfunction in Developmental Biology Metabolism)
►▼
Show Figures
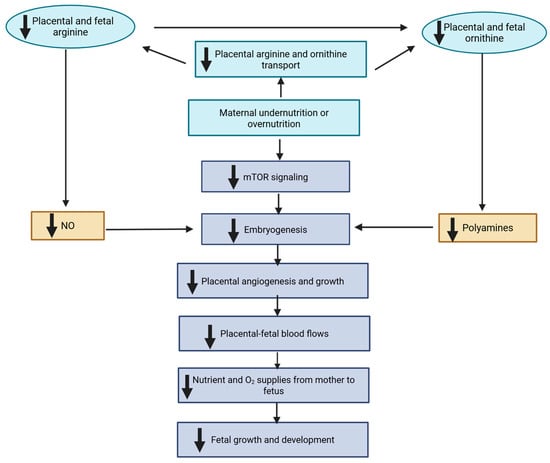
Figure 1
Open AccessArticle
A Zebrafish Seizure Model of cblX Syndrome Reveals a Dose-Dependent Response to mTor Inhibition
by
Claudia B. Gil, David Paz, Briana E. Pinales, Victoria L. Castro, Claire E. Perucho, Annalise Gonzales, Giulio Francia, Sepiso K. Masenga, Antentor Hinton, Jr. and Anita M. Quintana
J. Dev. Biol. 2026, 14(1), 2; https://doi.org/10.3390/jdb14010002 - 25 Dec 2025
Abstract
►▼
Show Figures
Mutations in the transcriptional co-factor HCFC1 cause methylmalonic aciduria and homocystinemia, cblX type (cblX) (MIM#309541), non-syndromic X-linked intellectual disability (XLID), and focal epilepsy. Zebrafish studies have revealed increased activation of the Akt/mTor signaling pathway after mutation of hcfc1a, one ortholog
[...] Read more.
Mutations in the transcriptional co-factor HCFC1 cause methylmalonic aciduria and homocystinemia, cblX type (cblX) (MIM#309541), non-syndromic X-linked intellectual disability (XLID), and focal epilepsy. Zebrafish studies have revealed increased activation of the Akt/mTor signaling pathway after mutation of hcfc1a, one ortholog of HCFC1. mTOR hyperactivation is linked to seizures, and its inhibition alleviates epilepsy in other preclinical models. We hypothesized that mTor overactivity in hcfc1a mutant zebrafish increases seizure susceptibility and/or severity. We employed a two-concentration model of the seizure-inducing agent, pentylenetetrazol (PTZ), with or without pretreatment of the mTor inhibitor, torin1. Mutation of hcfc1a did not alter the response to PTZ at sub-optimal concentrations, and the pharmaceutical inhibition of mTor using the compound Torin1 reduced response to 1 µM PTZ, but only in a dose-dependent manner. Higher doses of mTor inhibition did not reduce the seizure response in mutant larvae but were effective in wildtype siblings. These data suggest that inhibition of mTor in an hcfc1a-deficient background leads to a reaction that differs from the traditional response observed in wildtype siblings. Collectively, we present a model that can be used to test dose–response and the development of combinatorial treatment approaches in a high-throughput manner.
Full article

Graphical abstract
Open AccessArticle
The Epithelial Egg Tooth of the Chicken Shares Protein Markers with the Embryonic Subperiderm and Feathers
by
Attila Placido Sachslehner, Julia Steinbinder, Claudia Hess, Veronika Mlitz and Leopold Eckhart
J. Dev. Biol. 2026, 14(1), 1; https://doi.org/10.3390/jdb14010001 - 22 Dec 2025
Abstract
The epithelial egg tooth is used by birds to open the eggshell for hatching. This ectodermal structure consists of a multilayered periderm and a hard cornified portion, the caruncle or actual egg tooth. Here, we determined the protein composition of the egg tooth
[...] Read more.
The epithelial egg tooth is used by birds to open the eggshell for hatching. This ectodermal structure consists of a multilayered periderm and a hard cornified portion, the caruncle or actual egg tooth. Here, we determined the protein composition of the egg tooth of the chicken and compared the proteins to markers of other epithelia identified in previous studies. The egg tooth and the upper beak of chicken embryos of Hamburger and Hamilton (HH) stage 44 were subjected to mass spectrometry-based proteomics. We found that scaffoldin, a marker of the embryonic periderm and the feather sheath, was enriched in the egg tooth relative to the beak. Likewise, Epidermal Differentiation protein containing DPCC Motifs (EDDM) and Epidermal Differentiation protein starting with a MTF motif and rich in Histidine (EDMTFH), which had previously been characterized as markers of the subperiderm on embryonic scutate scales and the barbs of feathers, were also enriched in the egg tooth. The expression of EDDM and EDMTFH was confirmed RT-PCR analysis. Our data suggest that the epithelial egg tooth is related to the subperiderm and feathers, a hypothesis with potentially important implications for the evolution of the avian integument.
Full article
(This article belongs to the Special Issue Feature Papers from Journal of Developmental Biology Reviewers, 2nd Edition)
►▼
Show Figures

Figure 1
Open AccessReview
Pathophysiology and Management of Placenta Accreta Spectrum
by
Lana Shteynman, Genevieve Monanian, Gilberto Torres, Giancarlo Sabetta, Deborah M. Li, Zhaosheng Jin, Tiffany Angelo, Bahaa E. Daoud and Morgane Factor
J. Dev. Biol. 2025, 13(4), 45; https://doi.org/10.3390/jdb13040045 - 10 Dec 2025
Abstract
►▼
Show Figures
Placenta Accreta Spectrum (PAS) disorders, including placenta accreta, increta, and percreta, are serious obstetric conditions characterized by abnormal placental adherence to the uterine wall. With increasing incidence, PAS poses significant risks, primarily through massive hemorrhage during or after delivery, often necessitating hysterectomy. Key
[...] Read more.
Placenta Accreta Spectrum (PAS) disorders, including placenta accreta, increta, and percreta, are serious obstetric conditions characterized by abnormal placental adherence to the uterine wall. With increasing incidence, PAS poses significant risks, primarily through massive hemorrhage during or after delivery, often necessitating hysterectomy. Key risk factors include prior cesarean sections, uterine surgery, and placenta previa diagnosis. In this review, we will examine the pathophysiology of PAS, with a focus on the mechanisms underlying abnormal trophoblast invasion and defective decidualization. We will highlight the role of uterine scarring, extracellular matrix remodeling, dysregulated signaling pathways, and immune and vascular alterations in disrupting the maternal-fetal interface, ultimately predisposing to morbid placentation and delivery complications. We will also discuss the life-threatening complications of PAS, such as shock and multi-organ failure, which require urgent multidisciplinary intensive care, as well as the optimization of management through preoperative planning and intraoperative blood loss control to reduce maternal morbidity and mortality.
Full article

Figure 1
Open AccessSystematic Review
Cardiac Aftermath of Gestational Diabetes—From Intrauterine Impact to Lifelong Complications: A Systematic Review
by
Sophia Tsokkou, Ioannis Konstantinidis, Antonios Keramas, Vasileios Anastasiou, Alkis Matsas, Maria Florou, Alexandra Arvanitaki, Emmanouela Peteinidou, Theodoros Karamitsos, George Giannakoulas, Themistoklis Dagklis, Theodora Papamitsou, Antonios Ziakas and Vasileios Kamperidis
J. Dev. Biol. 2025, 13(4), 44; https://doi.org/10.3390/jdb13040044 - 8 Dec 2025
Abstract
►▼
Show Figures
Background. Gestational diabetes mellitus (GDM) induces maternal hyperglycemia, which may alter fetal cardiac structure and function, increasing short- and long-term cardiovascular risks. Purpose. To systematically review the evidence on the fetal cardiac structural and functional effects of GDM, to explore the
[...] Read more.
Background. Gestational diabetes mellitus (GDM) induces maternal hyperglycemia, which may alter fetal cardiac structure and function, increasing short- and long-term cardiovascular risks. Purpose. To systematically review the evidence on the fetal cardiac structural and functional effects of GDM, to explore the diagnostic role of novel imaging and biochemical biomarkers, and to summarize the long-term cardiovascular complications associated with GDM. Materials and Methods. A systematic search of PubMed, Scopus, and Cochrane Library was conducted according to the PRISMA guidelines. All studies comparing cardiac outcomes in GDM and non-GDM pregnancies were included. Data on myocardial hypertrophy, diastolic and systolic function, imaging modalities, and biomarkers were extracted and qualitatively synthesized. Results. A total of twelve eligible studies were identified. Fetal cardiac hypertrophy and diastolic and early systolic dysfunction are common among GDM pregnancies and can be detected by dual-gate Doppler and speckle-tracking echocardiography. Abnormalities are observed in indices such as the myocardial performance index, E/A, E/e′ ratios, and global longitudinal and circumferential strain in fetuses and may persist in the neonatal period. Alterations may be more pronounced for the right ventricle compared to the left. Septal hypertrophy is associated with elevated umbilical cord pro-brain natriuretic peptide. The risk of early-onset cardiovascular disease in the progeny of diabetic mothers is 29% higher, as evidenced by population-based cohort data. Conclusions. GDM is linked to fetal cardiac remodeling and an increased long-term cardiovascular risk. Early detection and customized interventions to reduce adverse outcomes may be achieved by integrating advanced echocardiographic techniques and biomarkers into prenatal surveillance.
Full article
Figure 1
Open AccessReview
How Cytoskeletal Disorders Contribute to Errors in the Chromosomal Segregation of Oocytes and Cleavage Stage Embryos
by
Stefka Delimitreva and Irina Chakarova
J. Dev. Biol. 2025, 13(4), 43; https://doi.org/10.3390/jdb13040043 - 2 Dec 2025
Abstract
►▼
Show Figures
Observations of the processes of oogenesis, fertilization, and the earliest embryonic development have given us the opportunity to estimate the importance of chromosomal distribution errors for the success of mammalian reproduction. It is now known that in the large volume of oocytes, zygotes
[...] Read more.
Observations of the processes of oogenesis, fertilization, and the earliest embryonic development have given us the opportunity to estimate the importance of chromosomal distribution errors for the success of mammalian reproduction. It is now known that in the large volume of oocytes, zygotes and the first embryonic cells, the rearrangement of chromatin is associated with a complex rearrangement of cytoskeletal structures, which creates specific problems. This review discusses two main issues critical to the success of early embryos: Why oocyte meiosis is too frequently wrong in chromosomal segregation? Why the first zygotic mitoses are too frequently wrong in chromosomal segregation? We concluded the following: (1) The main cytoskeletal defects that disturb oocyte meiosis are a problematic connection between cytoskeleton and nucleoskeleton, unsuccessful movement of the spindle to the oocyte periphery, unstable anchoring of the spindle to oolemma, and deviations in meiotic spindle morphology; (2) The main cytoskeletal defects that disturb pronuclear unification are nonfunctional male centriole, unsuccessful forming of microtubule aster around the sperm centrosome, problematic movement of the two pronuclei towards each other and inappropriate contacts between centrosomes, microtubules and nuclear pore complexes; (3) Cytoskeletal defects that disturb zygote mitosis are unsuccessful forming of bipolar mitotic spindle, non-synchronized congression of maternal and paternal chromosomes, and unsuccessful attachment of kinetochores to microtubules.
Full article

Figure 1
Open AccessArticle
Dynamic Alterations in Testicular Autophagy in Prepubertal Mice
by
Dong Zhang, Xiaoyun Pang, Zhenxing Yan, Weitao Dong, Zihao Fang, Jincheng Yang, Yanyan Wang, Li Xue, Jiahao Zhang, Chen Xue, Hongwei Duan, Xianghong Du and Yuxuan He
J. Dev. Biol. 2025, 13(4), 42; https://doi.org/10.3390/jdb13040042 - 18 Nov 2025
Abstract
►▼
Show Figures
Autophagy has a potential regulatory effect on spermatogenesis and testicular development. Dynamic alterations in the testicular autophagy of prepubertal mice were analyzed, and the relationship between autophagy levels and testicular development was clarified using C57BL/6 mice aged 1, 2, 4, 6, and 8
[...] Read more.
Autophagy has a potential regulatory effect on spermatogenesis and testicular development. Dynamic alterations in the testicular autophagy of prepubertal mice were analyzed, and the relationship between autophagy levels and testicular development was clarified using C57BL/6 mice aged 1, 2, 4, 6, and 8 weeks. Transmission electron microscopy was used to identify autophagic vacuoles. The expression of autophagy-related proteins and PI3K/AKT/mTOR signaling pathway-related proteins was determined using Western blotting. Localization of microtubule-associated protein light chain 3 (LC3) and sequestosome 1 (p62) in testicular tissues was determined using immunofluorescence and immunohistochemistry. Autophagic vacuoles in spermatogenic cells increased gradually from weeks 1 to 4, peaked at 2 weeks, decreased sharply at 6 weeks, and were undetectable at 8 weeks. The expression of Beclin 1 autophagy-related protein, LC3-II, and p62 was highest at 2 weeks among the five age groups, whereas LC3-II and p62 were mainly localized in spermatogonia and spermatocytes. Moreover, low mTOR expression and its increased expression were detected at 1–2 weeks and 2–8 weeks, respectively. These results show that testicular autophagic levels exhibit a dynamic pattern of “increase (1–2 weeks) followed by a decrease (2–8 weeks),” providing a reference in determining the relationship between autophagy levels and testicular development.
Full article

Figure 1
Open AccessArticle
Cloned Pig Fetuses Have a High Placental Lysophosphatidylcholine Level That Inhibits Trophoblast Cell Activity
by
Junkun Lai, Xiaoyu Gao, Guke Zhang, Xiao Wu, Yiqian Zhang, Shunbo Wang, Zhenfang Wu, Zicong Li and Zheng Xu
J. Dev. Biol. 2025, 13(4), 41; https://doi.org/10.3390/jdb13040041 - 12 Nov 2025
Abstract
►▼
Show Figures
Somatic cell nuclear transfer (SCNT) or cloning technology is widely used in agriculture and biomedicine. However, the application of this technology is limited by the low developmental competence of cloned embryos or fetuses, which frequently exhibit abnormal development of trophoblast cells or placentas.
[...] Read more.
Somatic cell nuclear transfer (SCNT) or cloning technology is widely used in agriculture and biomedicine. However, the application of this technology is limited by the low developmental competence of cloned embryos or fetuses, which frequently exhibit abnormal development of trophoblast cells or placentas. The purpose of this study was to investigate the possible causes of the erroneous placental development of SCNT-derived pig fetuses. The placental transcriptomic and lipidomic profiles were compared between 30-day-old SCNT- and artificial insemination (AI)-produced pig fetuses. Differentially expressed lipid metabolites between two groups of placentas were selected to test their effects on porcine trophoblast cell activity. The results showed that SCNT placentas exhibit impaired lipid metabolism and function. The level of a metabolite, lysophosphatidylcholine (LPC), in the glycerophospholipid metabolism pathway was substantially increased in SCNT placentas, compared with AI placentas. The elevation in LPC content may lead to impaired placental development in cloned pig fetuses, as LPC inhibited the proliferation and migration of porcine trophoblast cells. This study discovers a main cause of erroneous development of cloned pig fetuses, which will be beneficial for understanding the regulation of SCNT embryo development, as well as developing new methods to improve the efficiency of pig cloning.
Full article

Figure 1
Open AccessReview
Defective Neural Stem and Progenitor Cell Proliferation in Neurodevelopmental Disorders
by
Aki Shigenaka, Eri Nitta, Tadashi Nakagawa, Makiko Nakagawa and Toru Hosoi
J. Dev. Biol. 2025, 13(4), 40; https://doi.org/10.3390/jdb13040040 - 7 Nov 2025
Abstract
►▼
Show Figures
Neurodevelopmental disorders (NDDs), including autism spectrum disorder, intellectual disability, and attention deficit hyperactivity disorder, are increasingly recognized as disorders of early brain construction arising from defects in neural stem and progenitor cell (NSPC) proliferation. NSPCs are responsible for generating the diverse neuronal and
[...] Read more.
Neurodevelopmental disorders (NDDs), including autism spectrum disorder, intellectual disability, and attention deficit hyperactivity disorder, are increasingly recognized as disorders of early brain construction arising from defects in neural stem and progenitor cell (NSPC) proliferation. NSPCs are responsible for generating the diverse neuronal and glial lineages that establish cortical architecture and neural circuitry; thus, their expansion must be tightly coordinated by intrinsic cell cycle regulators and extrinsic niche-derived cues. Disruption of these mechanisms—through genetic mutations, epigenetic dysregulation, or environmental insults—can perturb the balance between NSPC self-renewal and differentiation, resulting in aberrant brain size and connectivity. Recent advances using animal models and human pluripotent stem cell-derived brain organoids have identified key signaling pathways, including Notch, Wnt, SHH, and PI3K–mTOR, as central hubs integrating proliferative cues, while transcriptional and chromatin regulators such as PAX6, CHD8, SETD5, and ANKRD11 govern gene expression essential for proper NSPC cycling. Furthermore, prenatal exposure to teratogens such as Zika virus infection, valproic acid, or metabolic stress in phenylketonuria can recapitulate proliferation defects and microcephaly, underscoring the vulnerability of NSPCs to environmental perturbation. This review summarizes emerging insights into the molecular and cellular mechanisms by which defective NSPC proliferation contributes to NDD pathogenesis, highlighting convergence among genetic and environmental factors on cell cycle control. A deeper understanding of these pathways may uncover shared therapeutic targets to restore neurodevelopmental trajectories and mitigate disease burden.
Full article

Figure 1
Open AccessReview
Recapitulating Liver Embryology—Lessons to Be Learned for Liver Diseases
by
Rui Caetano Oliveira, Sandra Ferreira, Isabel Gonçalves and Maria Fátima Martins
J. Dev. Biol. 2025, 13(4), 39; https://doi.org/10.3390/jdb13040039 - 4 Nov 2025
Abstract
►▼
Show Figures
Despite looking monotonous, liver histology represents a highly complex structure of hepatocytes, bile ducts and vessels. This complex interaction and development originate in embryology and remain in adult life. In this manuscript, we highlight the features of liver embryology, translating the events into
[...] Read more.
Despite looking monotonous, liver histology represents a highly complex structure of hepatocytes, bile ducts and vessels. This complex interaction and development originate in embryology and remain in adult life. In this manuscript, we highlight the features of liver embryology, translating the events into pathologic features and opening possibilities for disease understanding and research. We revisit liver embryology, from biliary to vascular processes, stressing some developing abnormalities with a focus on the histological findings. With this manuscript, we hope to increase the awareness of the importance of embryology in diseases, prompting its detailed study.
Full article

Figure 1
Open AccessArticle
Activity-Dependent Increases in Quantal Size at the Drosophila NMJ
by
Andrew S. Powers, Petar Gajic, Ethan Rittereiser, Kavindra Dasrat and Gregory A. Lnenicka
J. Dev. Biol. 2025, 13(4), 38; https://doi.org/10.3390/jdb13040038 - 28 Oct 2025
Abstract
We examined whether an increase in synaptic activity resulted in an increase in quantal size at the neuromuscular junction (NMJ) of third-instar Drosophila larvae. Spontaneous miniature excitatory postsynaptic currents (mEPSCs) or miniature excitatory postsynaptic potentials (mEPSPs) were recorded before and after nerve stimulation.
[...] Read more.
We examined whether an increase in synaptic activity resulted in an increase in quantal size at the neuromuscular junction (NMJ) of third-instar Drosophila larvae. Spontaneous miniature excitatory postsynaptic currents (mEPSCs) or miniature excitatory postsynaptic potentials (mEPSPs) were recorded before and after nerve stimulation. We found that prolonged (60 s) or brief (1.25 s) nerve stimulation produced an increase in quantal size; this appears to be a general property of these synapses since it was seen at all four muscle fibers (MFs) used in this study. The effect was examined along Is and Ib terminals by expressing GCaMP in the MF membrane and examining postsynaptic Ca2+ signals produced by spontaneous transmitter release. The activity-dependent increase in quantal size occurred at both Is and Ib terminals, and the increase in frequency and amplitude of quantal events at individual synaptic boutons was correlated. Both the increase in quantal size and frequency were found to be dependent upon an increase in postsynaptic Ca2+, based on studies in which MFs were preinjected with the Ca2+ chelator BAPTA (1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid). To examine the effect of postsynaptic activity on glutamate sensitivity, we iontophoresed glutamate pulses at the NMJ and recorded the glutamate-evoked excitatory postsynaptic potentials (gEPSPs). Trains of glutamate pulses produced an increase in gEPSP amplitude; this potentiation was not seen when Ca2+ was eliminated from the bath or after inhibiting calmodulin or CaMKII. The activity-dependent increase in quantal size may result from an increase in postsynaptic sensitivity due to activation of CaMKII.
Full article
(This article belongs to the Special Issue Drosophila in Developmental Biology—Past, Present and Future)
►▼
Show Figures

Figure 1
Open AccessArticle
A Supra-Physiological Dose of 2-Hydroxyestradiol Impairs Meiotic Progression and Developmental Competence of Mouse Antral Oocytes
by
Valeria Merico, Paola Rebuzzini, Mario Zanoni, Maurizio Zuccotti and Silvia Garagna
J. Dev. Biol. 2025, 13(4), 37; https://doi.org/10.3390/jdb13040037 - 15 Oct 2025
Abstract
►▼
Show Figures
Estrogen metabolites (EMs) play a local regulatory role in mammalian ovarian function. Among them, 2-hydroxyestradiol (2-OHE2) exerts dose-dependent effects on reproductive physiology, supporting either normal ovarian processes or contributing to pathological conditions. Specifically, 2-OHE2 modulates ovarian vasculature and progesterone biosynthesis, and at 1–10
[...] Read more.
Estrogen metabolites (EMs) play a local regulatory role in mammalian ovarian function. Among them, 2-hydroxyestradiol (2-OHE2) exerts dose-dependent effects on reproductive physiology, supporting either normal ovarian processes or contributing to pathological conditions. Specifically, 2-OHE2 modulates ovarian vasculature and progesterone biosynthesis, and at 1–10 nM concentrations, it enhances in vitro developmental competence and blastocyst quality in mouse oocytes. Conversely, doses below 1 nM show no appreciable effects, suggesting the existence of a biological activity threshold. However, the impact of supra-physiological concentrations remains largely unexplored. In this study, we investigated the effects of increasing 2-OHE2 doses (0.05, 0.50, and 5.00 µM) on oocyte meiotic progression and quality. Exposure to 0.50 and 5.00 µM significantly impaired oocyte maturation, while only the highest dose notably reduced the percentage of embryos developing to the blastocyst stage. Morphometric analysis during the GV-to-MII transition revealed altered first polar body morphology, defective asymmetric division, and disruptions in cytoskeletal organization, including enlarged meiotic spindles, increased F-actin cap angles, and aberrant microtubule-organizing centers distribution. These structural alterations were paralleled by distinct changes in cytoplasmic movement velocity patterns observed through time-lapse imaging during meiotic resumption. Together, these findings demonstrate that supra-physiological exposure to 2-OHE2 compromises oocyte maturation and developmental competence by perturbing key cytoskeletal dynamics and cellular architecture necessary for successful meiosis and early embryogenesis.
Full article

Figure 1
Open AccessReview
An Integrated Canonical and Non-Canonical Wnt Signaling Network Controls Early Anterior–Posterior Axis Formation in Sea Urchin Embryos
by
Jennifer L. Fenner, Boyuan Wang, Cheikhouna Ka, Sujan Gautam and Ryan C. Range
J. Dev. Biol. 2025, 13(4), 36; https://doi.org/10.3390/jdb13040036 - 8 Oct 2025
Abstract
►▼
Show Figures
Wnt signaling is an ancient developmental mechanism that drives the initial specification and patterning of the primary axis in many metazoan embryos. Yet, it is unclear how exactly the various Wnt components interact in most Wnt-mediated developmental processes as well as in the
[...] Read more.
Wnt signaling is an ancient developmental mechanism that drives the initial specification and patterning of the primary axis in many metazoan embryos. Yet, it is unclear how exactly the various Wnt components interact in most Wnt-mediated developmental processes as well as in the molecular mechanism regulating adult tissue homeostasis. Recent work in invertebrate deuterostome sea urchin embryos indicates that three different Wnt signaling pathways (Wnt/β-catenin, Wnt/JNK, and Wnt/PKC) form an interconnected Wnt signaling network that specifies and patterns the primary anterior–posterior (AP) axis. Here, we detail our current knowledge of this critical regulatory process in sea urchin embryos. We also illustrate examples from a diverse group of metazoans, from cnidarians to vertebrates, that suggest aspects of the sea urchin AP Wnt signaling network are deeply conserved. We explore how the sea urchin is an excellent model to elucidate a detailed molecular understanding of AP axis specification and patterning that can be used for identifying unifying developmental principles across animals.
Full article

Figure 1
Open AccessArticle
High Concentrations of Non-Esterified Fatty Acids During Bovine In Vitro Fertilisation Are Detrimental for Spermatozoa Quality and Pre-Implantation Embryo Development
by
Abdullah F. Idriss, Edward J. Okello, Roger G. Sturmey and Miguel A. Velazquez
J. Dev. Biol. 2025, 13(4), 35; https://doi.org/10.3390/jdb13040035 - 5 Oct 2025
Abstract
High non-esterified fatty acids (NEFAs) during negative energy balance in dairy cattle can impair reproduction. While their effects on oocyte maturation and preimplantation embryo development are known, their impact during fertilisation is largely unexplored. This study examined the effects of high NEFA exposure
[...] Read more.
High non-esterified fatty acids (NEFAs) during negative energy balance in dairy cattle can impair reproduction. While their effects on oocyte maturation and preimplantation embryo development are known, their impact during fertilisation is largely unexplored. This study examined the effects of high NEFA exposure exclusively during in vitro fertilisation (IVF). Bovine oocytes were matured in vitro and fertilised under physiological or high NEFA concentrations. High NEFA concentrations decreased fertilisation, cleavage, and blastocyst rates. Reactive oxygen species production in zygotes was not affected, but blastocysts derived from the High-NEFA group had fewer cells. Spermatozoa exposed to high NEFA concentrations exhibited increased plasma membrane and acrosome damage, higher DNA fragmentation, and reduced mitochondrial membrane potential. The expression of H3K27me3, a repressive histone mark normally erased from fertilisation to embryonic genome activation, was higher in 2-cell than in 4-cell embryos on day 2 after IVF, but only in the High-NEFA group. This delayed H3K27me3 loss, along with increased DNA damage, could partially explain the reduced blastocyst formation observed. In conclusion, high NEFA concentrations can impair pre-implantation embryo development during zygote formation, potentially via effects on both the oocyte and spermatozoon. The latter warrants further investigation using an intracytoplasmic sperm injection model.
Full article
(This article belongs to the Special Issue Embryonic Development and Regenerative Medicine)
►▼
Show Figures

Figure 1
Open AccessBrief Report
Exploring the Regulation of Tmem182 Gene Expression in the Context of Retinoid X Receptor Signaling
by
Saadia Khilji, Munerah Hamed, Jihong Chen and Qiao Li
J. Dev. Biol. 2025, 13(4), 34; https://doi.org/10.3390/jdb13040034 - 24 Sep 2025
Abstract
►▼
Show Figures
We have previously established that bexarotene, a clinically approved agonist of retinoid X receptor (RXR), promotes the differentiation and fusion of skeletal myoblasts. We have also analyzed the genomic programs underlying rexinoid-enhanced myogenic differentiation to identify novel regulatory pathways. As such, we observed
[...] Read more.
We have previously established that bexarotene, a clinically approved agonist of retinoid X receptor (RXR), promotes the differentiation and fusion of skeletal myoblasts. We have also analyzed the genomic programs underlying rexinoid-enhanced myogenic differentiation to identify novel regulatory pathways. As such, we observed a significant upregulation of a transcript encoding a predicted transmembrane protein, Tmem182, during C2C12 myoblast differentiation. Despite the documentation of Tmem182 expression in skeletal muscles, its regulation had yet to be explored. Here, we show that Tmem182 gene expression is markedly augmented in early myoblast differentiation and further enhanced by RXR signaling. In addition, Tmem182 expression is specific to muscle tissues and related to muscle master regulator MyoD. We found that MyoD and histone acetyltransferase p300 are bound to the Tmem182 promoter, and Tmem182 expression is p300-dependent. Thus, our data display a putative epigenetic signature associated with p300 and histone acetylation in rexinoid-responsive locus activation and transcription of myogenic targets.
Full article

Figure 1
Open AccessReview
Signaling Pathways in Human Blastocyst Development: From Molecular Mechanisms to In Vitro Optimization
by
Yan Jiao, Jiapeng Liu, Congge Li, Yuexin Hu and Sanjun Zhao
J. Dev. Biol. 2025, 13(3), 33; https://doi.org/10.3390/jdb13030033 - 9 Sep 2025
Cited by 1
Abstract
In recent years, assisted reproductive technology (ART) has developed rapidly with the delay in reproductive age and the rise in infertility rates. During ART, blastocyst quality is a key factor affecting the rate of implantation and clinical pregnancy, and blastocyst formation is dependent
[...] Read more.
In recent years, assisted reproductive technology (ART) has developed rapidly with the delay in reproductive age and the rise in infertility rates. During ART, blastocyst quality is a key factor affecting the rate of implantation and clinical pregnancy, and blastocyst formation is dependent on the precise regulation of multiple signaling pathways in preimplantation embryo development. In this review, we systematically analyze the molecular mechanisms of the core pathways, including Hippo, Wnt/β-catenin, FGF, Nodal, and BMP, in blastocyst lineage differentiation and morphogenesis, and assess the feasibility of optimizing in vitro culture by targeting key signaling nodes, as well as provide theoretical support for constructing research models of preimplantation embryos.
Full article
(This article belongs to the Collection Hedgehog Signaling in Embryogenesis)
►▼
Show Figures

Figure 1
Open AccessArticle
Zebrafish Unga Is Required for Genomic Maintenance upon Genotoxic Stress and Male Fertility
by
Latifa Kazzazy, Flóra Huba, Bálint Lóránt Hausz, Dávid Mező, Viktória Perey-Simon, Bálint Jezsó, Abdulrahman Seddik, Zoran Marinović, Judit Tóth, Angéla Békési, Beáta G. Vértessy and Máté Varga
J. Dev. Biol. 2025, 13(3), 32; https://doi.org/10.3390/jdb13030032 - 2 Sep 2025
Abstract
DNA repair is a multifaceted biological process that involves multiple pathways to counter the types of damage the genome encounters throughout life. In the past decade zebrafish became a popular model organism to study various aspects of vertebrate DNA repair, and the characterization
[...] Read more.
DNA repair is a multifaceted biological process that involves multiple pathways to counter the types of damage the genome encounters throughout life. In the past decade zebrafish became a popular model organism to study various aspects of vertebrate DNA repair, and the characterization of several mutant lines deficient in key players of the repair pathways has significantly contributed to our understanding of the roles the corresponding proteins play in the maintenance of genomic integrity. Interestingly, the base-excision repair (BER) pathway remained one of the less characterized DNA repair processes in fish. Here we provide a detailed characterization of zebrafish deficient in one of the key components of BER, the uracil-DNA glycosylase Unga. We show that while these fish are viable, they display an altered response to genotoxic stress and unga mutant males show an interesting form of subfertility.
Full article
(This article belongs to the Special Issue Zebrafish—a Model System for Developmental Biology Study III)
►▼
Show Figures

Figure 1
Open AccessReview
Profilin and Non-Canonical Wnt Signaling: Coordinating Cytoskeletal Dynamics from Development to Disease
by
Samira Alam, Danielle Duncan and Sharmin Hasan
J. Dev. Biol. 2025, 13(3), 31; https://doi.org/10.3390/jdb13030031 - 1 Sep 2025
Abstract
►▼
Show Figures
Vertebrate embryonic development relies on tightly regulated signaling pathways that guide morphogenesis, cell fate specification, and tissue organization. Among these, the Wnt signaling pathway plays a central role, orchestrating key developmental events. The non-canonical Wnt pathways, including the Planar Cell Polarity and Wnt/Ca
[...] Read more.
Vertebrate embryonic development relies on tightly regulated signaling pathways that guide morphogenesis, cell fate specification, and tissue organization. Among these, the Wnt signaling pathway plays a central role, orchestrating key developmental events. The non-canonical Wnt pathways, including the Planar Cell Polarity and Wnt/Ca2+ branches, are especially critical for regulating cytoskeletal dynamics during gastrulation. Recent studies highlight that these pathways interface with cytoskeletal effectors to control actin remodeling in response to extracellular cues. One such effector is Profilin, a small, evolutionarily conserved actin-binding protein that modulates actin polymerization and cellular architecture. Profilins, particularly Profilin1 and 2, are known to interact with Daam1, a formin protein downstream of PCP signaling, thereby linking Wnt signals to actin cytoskeletal regulation. Emerging evidence suggests that Profilins are active signaling intermediates that contribute to morphogenetic processes. Their context-dependent interactions and differential expression across species also suggest that they play specialized roles in development and disease. This review synthesizes the current understanding of Profilin’s role in non-canonical Wnt signaling, examining its molecular interactions and contributions to cytoskeletal control during development. By integrating data across model systems, we aim to clarify how Profilins function at the intersection of signaling and cytoskeletal dynamics, with implications for both developmental biology and disease pathogenesis.
Full article

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Special Issues
Special Issue in
JDB
Cilia in Development: Second Edition
Guest Editor: Aimin LiuDeadline: 31 January 2026
Special Issue in
JDB
Developmental Biology of the Kidney: From Molecular Mechanisms to Congenital Disorders
Guest Editors: Katarina Vukojević, Anita RacetinDeadline: 31 January 2026
Special Issue in
JDB
Mitochondrial Function and Dysfunction in Developmental Biology Metabolism
Guest Editor: Simon J. ConwayDeadline: 15 March 2026
Special Issue in
JDB
Feature Papers from Journal of Developmental Biology Reviewers, 2nd Edition
Guest Editors: Christopher A. Johnston, Junichi IwataDeadline: 25 March 2026
Topical Collections
Topical Collection in
JDB
Hedgehog Signaling in Embryogenesis
Collection Editors: Henk Roelink, Kay Grobe









