Mechanistic and Structural Studies of Protein-Only RNase P Compared to Ribonucleoproteins Reveal the Two Faces of the Same Enzymatic Activity
Abstract
:1. Introduction
2. Diversity of PRORP Sequence Features
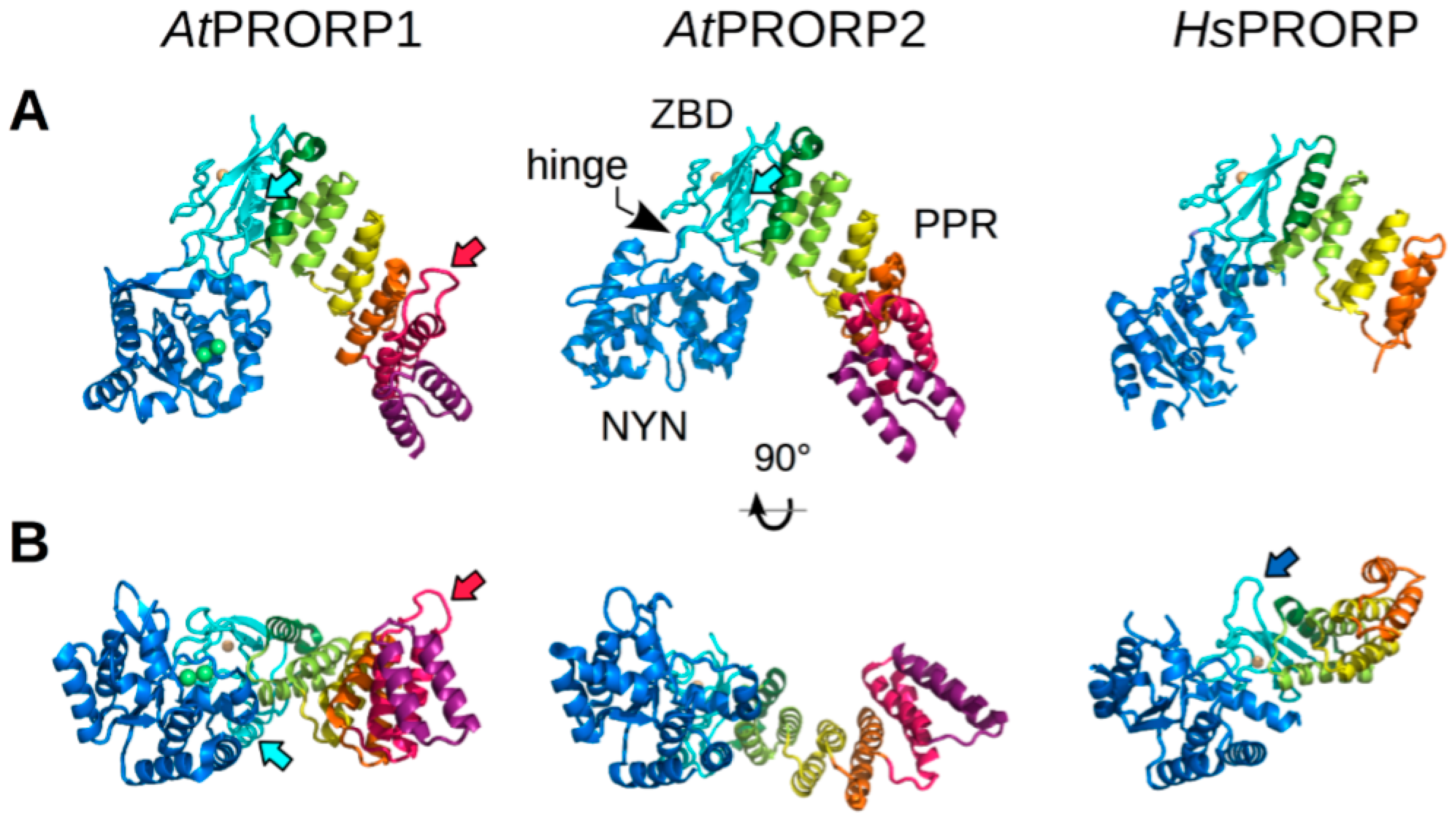
3. Comparison of PRORP Three-Dimensional Structures
3.1. Common Structural Features
3.2. Specific Structural Features
4. Mechanistic Analyses of Protein-Only RNase P Activity
4.1. Kinetic Analyses of PRORP Activity
4.2. Involvement of PRORP cis-Elements for RNase P Activity
4.3. Requirements of Pre-tRNA Substrate cis-elements for PRORP Activity
4.4. PRORP Cleavage Mechanism
5. Comparison of PRORP and RNP RNase P Modes of Action
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NYN | N4BP1, YacP-like Nuclease |
| PPR | pentatricopeptide repeat |
| P-RNA | ribonucleoprotein RNase P RNA subunit |
| Pre-tRNA | precursor tRNA |
| PRORP | protein-only RNase P |
| RNP | ribonucleoprotein |
| tRNA | transfer RNA |
References
- Altman, S. A view of RNase P. Mol. Biosyst. 2007, 3, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Davis, N.W.; Gegenheimer, P. Novel mechanisms for maturation of chloroplast transfer RNA precursors. EMBO J. 1988, 7, 1567–1574. [Google Scholar] [PubMed]
- Rossmanith, W.; Karwan, R.M. Characterization of human mitochondrial RNase P: Novel aspects in tRNA processing. Biochem. Biophys. Res. Commun. 1998, 247, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Salavati, R.; Panigrahi, A.K.; Stuart, K.D. Mitochondrial ribonuclease P activity of Trypanosoma brucei. Mol. Biochem. Parasitol. 2001, 115, 109–117. [Google Scholar] [CrossRef]
- Pinker, F.; Bonnard, G.; Gobert, A.; Gutmann, B.; Hammani, K.; Sauter, C.; Gegenheimer, P.A.; Giegé, P. PPR proteins shed a new light on RNase P biology. RNA Biol. 2013, 10, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Guerrier-Takada, C.; Gardiner, K.; Marsh, T.; Pace, N.; Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 1983, 35, 849–857. [Google Scholar] [CrossRef]
- Guerrier-Takada, C.; Altman, S. Catalytic activity of an RNA molecule prepared by transcription in vitro. Science 1984, 223, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Guerrier-Takada, C.; Lumelsky, N.; Altman, S. Specific interactions in RNA enzyme-substrate complexes. Science 1989, 246, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Randau, L.; Schroder, I.; Söll, D. Life without RNase P. Nature 2008, 453, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.; Hartmann, R.K. The enigma of ribonuclease P evolution. Trends Genet. 2003, 19, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Rossmanith, W.; Hartmann, R.K.; Tholken, C.; Gutmann, B.; Giegé, P.; Gobert, A. Distribution of Ribonucleoprotein and Protein-Only RNase P in Eukarya. Mol. Biol. Evol. 2015, 32, 3186–3193. [Google Scholar] [CrossRef] [PubMed]
- Seif, E.; Leigh, J.; Liu, Y.; Roewer, I.; Forget, L.; Lang, B.F. Comparative mitochondrial genomics in zygomycetes: Bacteria-like RNase P RNAs, mobile elements and a close source of the group i intron invasion in angiosperms. Nucleic Acids Res. 2005, 33, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Ellis, F.C.; Brown, J.W. The evolution of RNase P and its RNA. In Ribonuclease P; Liu, F., Altman, S., Eds.; Springer: New York, NY, USA, 2010; pp. 17–40. [Google Scholar]
- Lai, L.B.; Vioque, A.; Kirsebom, L.A.; Gopalan, V. Unexpected diversity of RNase P, an ancient tRNA processing enzyme: Challenges and prospects. FEBS Lett. 2010, 584, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.B.; Cho, I.-M.; Chen, W.-Y.; Gopalan, V. Archaeal RNase P: A Mosaic of Its Bacterial and Eukaryal Relatives. In Ribonuclease P; Liu, F., Altman, S., Eds.; Springer: New York, NY, USA, 2010; pp. 153–172. [Google Scholar]
- Walker, S.C.; Marvin, M.C.; Engelke, D.R. Eukaryote RNase P and RNase MRP. In Ribonuclease P; Liu, F., Altman, S., Eds.; Springer: New York, NY, USA, 2010; pp. 173–202. [Google Scholar]
- Anantharaman, V.; Aravind, L. The NYN domains: Novel predicted rnases with a pin domain-like fold. RNA Biol. 2006, 3, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Giegé, P. Pentatricopeptide repeat proteins: A set of modular rna-specific binders massively used for organelle gene expression. RNA Biol. 2013, 10, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.J.; Lim, W.H.; Fierke, C.A.; Koutmos, M. Mitochondrial ribonuclease P structure provides insight into the evolution of catalytic strategies for precursor-tRNA 5′ processing. Proc. Natl. Acad. Sci. USA 2012, 109, 16149–16154. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Pinker, F.; Fuchsbauer, O.; Gutmann, B.; Boutin, R.; Roblin, P.; Sauter, C.; Giegé, P. Structural insights into protein-only RNase P complexed with trna. Nat. Commun. 2013, 4, 1353. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, J.; Frank, P.; Loffler, E.; Bennett, K.L.; Gerner, C.; Rossmanith, W. RNAse P without RNA: Identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell 2008, 135, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Gutmann, B.; Taschner, A.; Gößringer, M.; Holzmann, J.; Hartmann, R.K.; Rossmanith, W.; Giegé, P. A single Arabidopsis organellar protein has RNase P activity. Nat. Struct. Mol. Biol. 2010, 17, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, B.; Gobert, A.; Giegé, P. Prorp proteins support RNase P activity in both organelles and the nucleus in Arabidopsis. Genes Dev. 2012, 26, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Täschner, A.; Weber, C.; Buzet, A.; Hartmann, R.K.; Hartig, A.; Rossmanith, W. Nuclear RNase P of Trypanosoma brucei: A single protein in place of the multicomponent RNA-protein complex. Cell Rep. 2012, 2, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.B.; Bernal-Bayard, P.; Mohannath, G.; Lai, S.M.; Gopalan, V.; Vioque, A. A functional RNase P protein subunit of bacterial origin in some eukaryotes. Mol. Genet. Genom. 2011, 286, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Sugita, C.; Komura, Y.; Tanaka, K.; Kometani, K.; Satoh, H.; Sugita, M. Molecular characterization of three PRORP proteins in the moss Physcomitrella patens: Nuclear prorp protein is not essential for moss viability. PLoS ONE 2014, 9, e108962. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, G.; Gobert, A.; Pinker, F.; Arrivé, M.; Salinas, T.; Giegé, P. A single gene encodes both organelles and nuclear RNase P enzymes in Chlamydomonas reinhardtii. Plant J. 2016, in press. [Google Scholar]
- Wang, G.; Chen, H.W.; Oktay, Y.; Zhang, J.; Allen, E.L.; Smith, G.M.; Fan, K.C.; Hong, J.S.; French, S.W.; McCaffery, J.M.; et al. PNPase regulates RNA import into mitochondria. Cell 2010, 142, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Rossmanith, W. Of P and Z: Mitochondrial tRNA processing enzymes. Biochim. Biophys. Acta 2012, 1819, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Hartig, A.; Hartmann, R.K.; Rossmanith, W. Playing RNase P evolution: Swapping the RNA catalyst for a protein reveals functional uniformity of highly divergent enzyme forms. PLoS Genet. 2014, 10, e1004506. [Google Scholar] [CrossRef] [PubMed]
- Serruya, R.; Orlovetskie, N.; Reiner, R.; Dehtiar-Zilber, Y.; Wesolowski, D.; Altman, S.; Jarrous, N. Human RNase P ribonucleoprotein is required for formation of initiation complexes of RNA polymerase iii. Nucleic Acids Res. 2015, 43, 5442–5450. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.; Rojas, M.; Fujii, S.; Yap, A.; Chong, Y.S.; Bond, C.S.; Small, I. A combinatorial amino acid code for RNA recognition by pentatricopeptide repeat proteins. PLoS Genet. 2012, 8, e1002910. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Zehrmann, A.; Brennicke, A.; Graichen, K. Improved computational target site prediction for pentatricopeptide repeat RNA editing factors. PLoS ONE 2013, 8, e65343. [Google Scholar] [CrossRef] [PubMed]
- Yagi, Y.; Hayashi, S.; Kobayashi, K.; Hirayama, T.; Nakamura, T. Elucidation of the RNA recognition code for pentatricopeptide repeat proteins involved in organelle RNA editing in plants. PLoS ONE 2013, 8, e57286. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Gutmann, B.; Zhong, X.; Ye, Y.; Fisher, M.F.; Bai, F.; Castleden, I.; Song, Y.; Song, B.; Huang, J.; et al. Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. Plant J. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, L.; Sridhara, S.; Hallberg, B.M. Structure of the nuclease subunit of human mitochondrial RNase P. Nucleic Acids Res. 2015, 43, 5664–5672. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.; Zhou, W.; Yang, X.; Shen, Y. Auto-inhibitory mechanism of the human mitochondrial RNase P protein complex. Sci. Rep. 2015, 5, 9878. [Google Scholar] [CrossRef] [PubMed]
- Pinker, F.; Giegé, P.; Sauter, C. Crystallization and crystallographic analysis of an Arabidopsis nuclear proteinaceous RNase P. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Karasik, A.; Shanmuganathan, A.; Howard, M.J.; Fierke, C.A.; Koutmos, M. Nuclear protein-only ribonuclease P2 structure and biochemical characterization provide insight into the conserved properties of tRNA 5′ end processing enzymes. J. Mol. Biol. 2016, 428, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Small, I.D.; Peeters, N. The PPR motif—A TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 2000, 25, 46–47. [Google Scholar] [CrossRef]
- Zeytuni, N.; Zarivach, R. Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure 2012, 20, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Ringel, R.; Sologub, M.; Morozov, Y.I.; Litonin, D.; Cramer, P.; Temiakov, D. Structure of human mitochondrial RNA polymerase. Nature 2011, 478, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Li, Q.; Yan, C.; Liu, Y.; Liu, J.; Yu, F.; Wang, Z.; Long, J.; He, J.; Wang, H.W.; et al. Structural basis for the modular recognition of single-stranded RNA by PPR proteins. Nature 2013, 504, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Chen, R.Z.; Ban, T.; Zhou, X.E.; Gu, X.; Tan, M.H.; Chen, C.; Kang, Y.; Brunzelle, J.S.; Zhu, J.K.; et al. Structural basis for RNA recognition by a dimeric PPR protein complex. Nat. Struct. Mol. Biol. 2013, 20, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.; Ke, J.; Chen, R.; Gu, X.; Tan, M.H.; Zhou, X.E.; Kang, Y.; Melcher, K.; Zhu, J.K.; Xu, H.E. Structure of a PLS-class pentatricopeptide repeat protein provides insights into mechanism of RNA recognition. J. Biol. Chem. 2013, 288, 31540–31548. [Google Scholar] [CrossRef] [PubMed]
- Glavan, F.; Behm-Ansmant, I.; Izaurralde, E.; Conti, E. Structures of the PIN domains of SMG6 and SMG5 reveal a nuclease within the mRNA surveillance complex. EMBO J. 2006, 25, 5117–5125. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Peng, W.; Sun, Y.; Wang, X.; Xu, Y.; Li, X.; Gao, G.; Rao, Z. Structural study of mcpip1 N-terminal conserved domain reveals a PIN-like RNase. Nucleic Acids Res. 2012, 40, 6957–6965. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, M.; Meara, D.; Nossal, N.G. Identification of residues of T4 RNase H required for catalysis and DNA binding. J. Biol. Chem. 1997, 272, 28531–28538. [Google Scholar] [CrossRef] [PubMed]
- Gully, B.S.; Cowieson, N.; Stanley, W.A.; Shearston, K.; Small, I.D.; Barkan, A.; Bond, C.S. The solution structure of the pentatricopeptide repeat protein PPR10 upon binding atph RNA. Nucleic Acids Res 2015, 43, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Giegé, T.; Giegé, R.; Giegé, P. tRNA biology in mitochondria. Int. J. Mol. Sci. 2015, 16, 4518–4559. [Google Scholar] [CrossRef] [PubMed]
- Vilardo, E.; Nachbagauer, C.; Buzet, A.; Taschner, A.; Holzmann, J.; Rossmanith, W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase-extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012, 40, 11583–11593. [Google Scholar] [CrossRef] [PubMed]
- LaGrandeur, T.E.; Darr, S.C.; Haas, E.S.; Pace, N.R. Characterization of the RNase P RNA of Sulfolobus acidocaldarius. J. Bacteriol. 1993, 175, 5043–5048. [Google Scholar] [PubMed]
- Pan, T.; Loria, A.; Zhong, K. Probing of tertiary interactions in RNA: 2′-hydroxyl-base contacts between the RNase P RNA and pre-tRNA. Proc. Natl. Acad. Sci. USA 1995, 92, 12510–12514. [Google Scholar] [CrossRef] [PubMed]
- Reiter, N.J.; Osterman, A.; Torres-Larios, A.; Swinger, K.K.; Pan, T.; Mondragon, A. Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA. Nature 2010, 468, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Kurz, J.C.; Niranjanakumari, S.; Fierke, C.A. Protein component of Bacillus subtilis RNase P specifically enhances the affinity for precursor-tRNAAsp. Biochemistry 1998, 37, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Brillante, N.; Gossringer, M.; Lindenhofer, D.; Toth, U.; Rossmanith, W.; Hartmann, R.K. Substrate recognition and cleavage-site selection by a single-subunit protein-only RNase P. Nucleic Acids Res. 2016, 44, 2323–2336. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.J.; Karasik, A.; Klemm, B.P.; Mei, C.; Shanmuganathan, A.; Fierke, C.A.; Koutmos, M. Differential substrate recognition by isozymes of plant protein-only ribonuclease P. RNA 2016, 22, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Nakamura, T.; Maeda, T.; Nakayama, K.; Gao, X.; Nakashima, T.; Kakuta, Y.; Kimura, M. Pentatricopeptide repeat motifs in the processing enzyme PRORP1 in Arabidopsis thaliana play a crucial role in recognition of nucleotide bases at TYC loop in precursor tRNAs. Biochem. Biophys. Res. Commun. 2014, 450, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Tanimoto, A.; Shkriabai, N.; Kvaratskhelia, M.; Wysocki, V.; Gopalan, V. Use of chemical modification and mass spectrometry to identify substrate-contacting sites in proteinaceous RNase P, a tRNA processing enzyme. Nucleic Acids Res. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, L.V.; Gossringer, M.; Weber, C.; Buzet, A.; Rossmanith, W.; Hartmann, R.K. tRNA processing by protein-only versus RNA-based RNase P: Kinetic analysis reveals mechanistic differences. Chembiochem 2012, 13, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Steitz, T.A.; Steitz, J.A. A general two-metal-ion mechanism for catalytic RNA. Proc. Natl. Acad. Sci. USA 1993, 90, 6498–6502. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.C.; Li, X.; Gegenheimer, P. Chloroplast ribonuclease P does not utilize the ribozyme-type pre-tRNA cleavage mechanism. RNA 2000, 6, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Donghi, D.; Schnabl, J. Multiple roles of metal ions in large ribozymes. Met. Ions Life Sci. 2011, 9, 197–234. [Google Scholar] [PubMed]
- Howard, M.J.; Klemm, B.P.; Fierke, C.A. Mechanistic studies reveal similar catalytic strategies for phosphodiester bond hydrolysis by protein-only and RNA-dependent ribonuclease P. J. Biol. Chem. 2015, 290, 13454–13464. [Google Scholar] [CrossRef] [PubMed]
- Tsonis, P.A.; Dwivedi, B. Molecular mimicry: Structural camouflage of proteins and nucleic acids. Biochim. Biophys. Acta 2008, 1783, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Giegé, R. Toward a more complete view of tRNA biology. Nat. Struct. Mol. Biol. 2008, 15, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Suzuki, T.; Giegé, P.; Higashiyama, T.; Koizuka, N.; Shikanai, T. The restorer-of-fertility-like 2 pentatricopeptide repeat protein and RNase P are required for the processing of mitochondrial orf291 RNA in Arabidopsis. Plant J. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
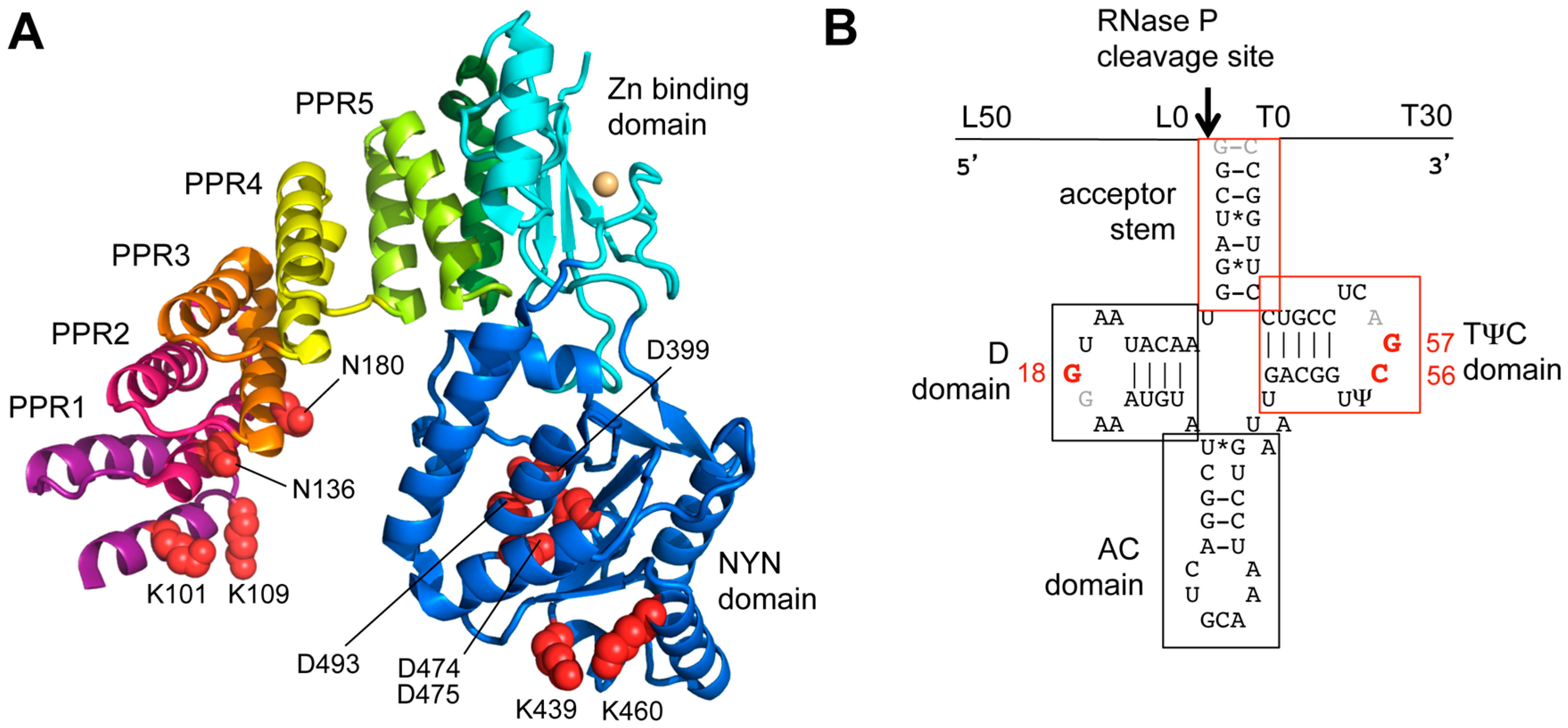
| RNase P | Domain Mutated | Position Mutated | pre-tRNA | Cleavage (+/− or %) | kobs (min−1) | kcat (min−1) | KM (nM) | KD (nM) | References |
|---|---|---|---|---|---|---|---|---|---|
| AtPRORP1 | - | At mito pre-tRNACys | + | [22] | |||||
| NYN | D474A/D475A | - | |||||||
| Nt deletion | Δ76 | 1.4 ± 0.1 | 700 ± 100 | [19] | |||||
| Nt deletion | Δ245 | <0.001 | 24,000 ± 10,000 | ||||||
| NYN | D399A | <0.001 | 1100 ± 100 | ||||||
| NYN | D474A | <0.001 | 1400 ± 600 | ||||||
| NYN | D475A | <0.001 | 1400 ± 100 | ||||||
| NYN | D493A | <0.001 | 300 ± 100 | ||||||
| - | 2.22 ± 0.12 | 3.72 ± 0.3 | 670 ± 230 | 510 ± 120 | [57] | ||||
| - | 1.2 ± 0.3 | 250 ± 34 | [59] | ||||||
| PPR 1 | K101A | 1.2 ± 0.4 | 859 ± 159 | ||||||
| PPR 1 | K109A | 1.3 ± 0.3 | 389 ± 35 | ||||||
| NYN | K439A | 0.4 ± 0.1 | 609 ± 70 | ||||||
| NYN | K460A | 1.3 ± 0.1 | 377 ± 23 | ||||||
| PPR 1 | K101A/K109A | 2.5 ± 0.1 | 1624 ± 344 | ||||||
| PPR 1/NYN | K101A/K439A | 0.5 ± 0.1 | 888 ± 127 | ||||||
| PPR 1/NYN | K109A/K439A | 0.5 ± 0.1 | 763 ± 92 | ||||||
| - | Tt pre-tRNAGly | 2.3 ± 0.6 | [60] | ||||||
| - | At chloro pre-tRNAPhe | + | [22] | ||||||
| NYN | D474A/D475A | - | |||||||
| - | 100 | [58] | |||||||
| PPR 2 | N136T | 60 | |||||||
| PPR 3 | T180N | 26 | |||||||
| PPR 4 | G215N | 85 | |||||||
| - | 100 | ||||||||
| Nt deletion | Δ89 | 35 | |||||||
| Domain deletion | ΔPPR1 | 3 | |||||||
| Domain deletion | ΔPPR1-2 | 0 | |||||||
| Domain deletion | ΔPPR1-3 | 0 | |||||||
| Domain deletion | Δ89 PPR2 | 0 | |||||||
| Domain deletion | Δ89 PPR3 | 0 | [57] | ||||||
| - | 2.1 ± 0.12 | 2.52 ± 0.3 | 140 ± 50 | 60 ± 10 | |||||
| - | At nuc pre-tRNACys | 2.22 ± 0.18 | 2.4 ± 0.12 | 550 ± 50 | 2300 ± 300 | ||||
| - | At nuc pre-tRNAPhe | 4.68 ± 0.18 | 2.1 ± 0.18 | 160 ± 50 | 330 ± 60 | ||||
| AtPRORP2 | - | Tt pre-tRNAGly | 5.0 ± 1.2 | [60] | |||||
| - | At nuc pre-tRNAGly 8:1 | 1.1 ± 0.1 | [39] | ||||||
| NYN | D393A | <0.001 | |||||||
| NYN | D421A | <0.001 | |||||||
| NYN | D422A | <0.001 | |||||||
| NYN | D440A | <0.001 | |||||||
| NYN | H445A | 0.02 ± 0.004 | |||||||
| Nt deletion | Δ141 | <0.001 | |||||||
| - | At mito pre-tRNACys | 0.78 ± 0.18 | 0.78 ± 0.12 | 340 ± 60 | 350 ± 70 | [57] | |||
| - | At chloro tRNAPhe | 1.08 ± 0.18 | 0.9 ± 0.12 | 340 ± 100 | 140 ± 10 | ||||
| - | At nuc pre-tRNACys | 1.62 ± 0.12 | 1.8 ± 0.12 | 940 ± 130 | 6100 ± 2100 | ||||
| - | At nuc pre-tRNAPhe | 2.1 ± 0.12 | 1.38 ± 0.12 | 250 ± 50 | 350 ± 40 | ||||
| AtPRORP3 | - | At pre-tRNAGln | 300 ± 90 | [23] | |||||
| - | Tt pre-tRNAGly | 7.7 ± 2.7 | [60] | ||||||
| - | Tt pre-tRNAGly | 1.8 ± 0.1 | [56] | ||||||
| PPR 3 | T113S | 2.0 ± 0.1 | |||||||
| PPR 3 | R145N | 2.0 ± 0.1 | |||||||
| PPR 3 | R145D | 1.15 ± 0.02 | |||||||
| PPR 3 | T113N | 1.56 ± 0.04 | |||||||
| PPR 3 | T113N-R145N | 0.38 ± 0.02 | |||||||
| PPR 3 | T113N-R145D | 0.047 ± 0.002 | |||||||
| - | At mito pre-tRNACys | 1.38 ± 0.12 | 1.32 ± 0.12 | 430 ± 30 | 300 ± 70 | [57] | |||
| - | At chloro pre-tRNAPhe | 1.38 ± 0.12 | 0.78 ± 0.12 | 440 ± 50 | 220 ± 30 | ||||
| - | At nuc pre-tRNACys | 1.80 ± 0.12 | 0.48 ± 0.12 | 420 ± 100 | 1500 ± 200 | ||||
| - | At nuc pre-tRNAPhe | 4.32 ± 0.18 | 3.72 ± 1.38 | 2000 ± 850 | 380 ± 50 |
| pre-tRNA | Type of Mutation on pre-tRNA | RNase P | % of Cleavage | kobs or kreact (min−1) | KM (nM) | KD (nM) | References |
|---|---|---|---|---|---|---|---|
| At mito pre-tRNACys | - | AtPRORP1 | 100 ± 7 | [20] | |||
| ΔAC | 75 ± 9 | ||||||
| ΔDAC | 0 ± 0 | ||||||
| G18A | 15 ± 2 | ||||||
| G18C | 10 ± 1 | ||||||
| G19A | 85 ± 3 | ||||||
| G19C | 90 ± 5 | ||||||
| C56A | 0 ± 0 | ||||||
| C56G | 0 ± 0 | ||||||
| G57A | 90 ± 6 | ||||||
| G57C | 10 ± 1 | ||||||
| 1CG72 | 100 ± 10 | ||||||
| Δ3′ | 95 ± 1 | ||||||
| 3′ CCA | 5 ± 2 | ||||||
| At chloro pre-tRNAPhe | - | 100 | [58] | ||||
| C56G | 18 | ||||||
| C56A | 30 | ||||||
| C56U | 22 | ||||||
| A57G | 60 | ||||||
| A57C | 25 | ||||||
| A57U | 26 | ||||||
| A58G | 15 | ||||||
| A58C | 36 | ||||||
| A58U | 35 | ||||||
| A59G | 100 | ||||||
| A59C | 75 | ||||||
| A59U | 85 | ||||||
| At nuc pre-tRNAGly | L23:T10 | AtPRORP2 | 0.7 ± 0.1 | 118 ± 26 | [39] | ||
| L23:T05 | 1.0 ± 0.1 | 52 ± 12 | |||||
| L23:T01 | 0.7 ± 0.1 | 17 ± 5 | |||||
| L13:T01 | 0.7 ± 0.1 | 6 ± 1 | |||||
| L08:T01 | 1.1 ± 0.1 | 3 ± 1 | |||||
| Tt pre-tRNAGly | −(14) | AtPRORP3 | 1.67 ± 0.03 | 4.8 ± 0.4 | [56] | ||
| L7 | 1.7 ± 0.1 | 3.1 ± 0.7 | |||||
| L4 | 1.7 ± 0.1 | 3.4 ± 0.7 | |||||
| L2 | 1.6 ± 0.1 | 3.4 ± 0.8 | |||||
| L1 | 0.17 ± 0.02 | 5.4 ± 2.2 | |||||
| mature (CCA) | 1.6 ± 0.1 | 4.9 ± 1.0 | |||||
| no trailer | 1.5 ± 0.1 | 4.6 ± 0.7 | |||||
| 40-nt trailer | 1.5 ± 0.1 | 5.3 ± 1.1 | |||||
| - | 1.67 ± 0.03 | 4.8 ± 0.4 | |||||
| U1-A72 | 2.2 ± 0.1 | 5.3 ± 0.9 | |||||
| U-1 | 2.9 ± 0.1 | 8.1 ± 1.4 | |||||
| G-1, A73 | 2.3 ± 0.1 | 6.5 ± 1.0 | |||||
| A-1, A73 | 5.1 ± 0.2 | 7.8 ± 1.4 | |||||
| A73 | 1.67 ± 0.04 | 4.5 ± 0.6 | |||||
| ΔAC | 1.48 ± 0.04 | 1.7 ± 0.3 | |||||
| ΔD | 0.36 ± 0.02 | 86 ± 16 | |||||
| AaT | 0.066 ± 0.002 | 1839 ± 168 | |||||
| Aab1T | 0.33 ± 0.01 | 1685 ± 218 | |||||
| Aab4T | 0.26 ± 0.01 | 1151 ± 125 | |||||
| Aab9T | 0.42 ± 0.01 | 40 ± 6 | |||||
| G18->A18 | 1.87 ± 0.07 | 22 ± 3 | |||||
| G19->A19/C56 > U56 | 1.78 ± 0.06 | 7.7 ± 1.2 | |||||
| C56->U56 | 1.81 ± 0.05 | 6.4 ± 0.9 | |||||
| A57->C57 | 1.56 ± 0.05 | 6.7 ± 0.9 | |||||
| Bs pre-tRNAAsp | L0 | AtPRORP1 | NA | 3400 ± 400 | [57] | ||
| L1 | 4.68 ± 0.18 | 150 ± 60 | |||||
| L2 | 9 ± 1.2 | 310 ± 20 | |||||
| L3 | 1.92 ± 0.06 | 140 ± 40 | |||||
| L4 | 1.5 ± 0.06 | 150 ± 40 | |||||
| L5 | 1.5 ± 0.06 | 190 ± 60 | |||||
| L10 | 1.5 ± 0.06 | 100 ± 50 | |||||
| L14 | 1.2 ± 0.06 | 100 ± 50 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schelcher, C.; Sauter, C.; Giegé, P. Mechanistic and Structural Studies of Protein-Only RNase P Compared to Ribonucleoproteins Reveal the Two Faces of the Same Enzymatic Activity. Biomolecules 2016, 6, 30. https://doi.org/10.3390/biom6030030
Schelcher C, Sauter C, Giegé P. Mechanistic and Structural Studies of Protein-Only RNase P Compared to Ribonucleoproteins Reveal the Two Faces of the Same Enzymatic Activity. Biomolecules. 2016; 6(3):30. https://doi.org/10.3390/biom6030030
Chicago/Turabian StyleSchelcher, Cédric, Claude Sauter, and Philippe Giegé. 2016. "Mechanistic and Structural Studies of Protein-Only RNase P Compared to Ribonucleoproteins Reveal the Two Faces of the Same Enzymatic Activity" Biomolecules 6, no. 3: 30. https://doi.org/10.3390/biom6030030






