Sulfatide-Hsp70 Interaction Promotes Hsp70 Clustering and Stabilizes Binding to Unfolded Protein
Abstract
:1. Introduction
2. Results
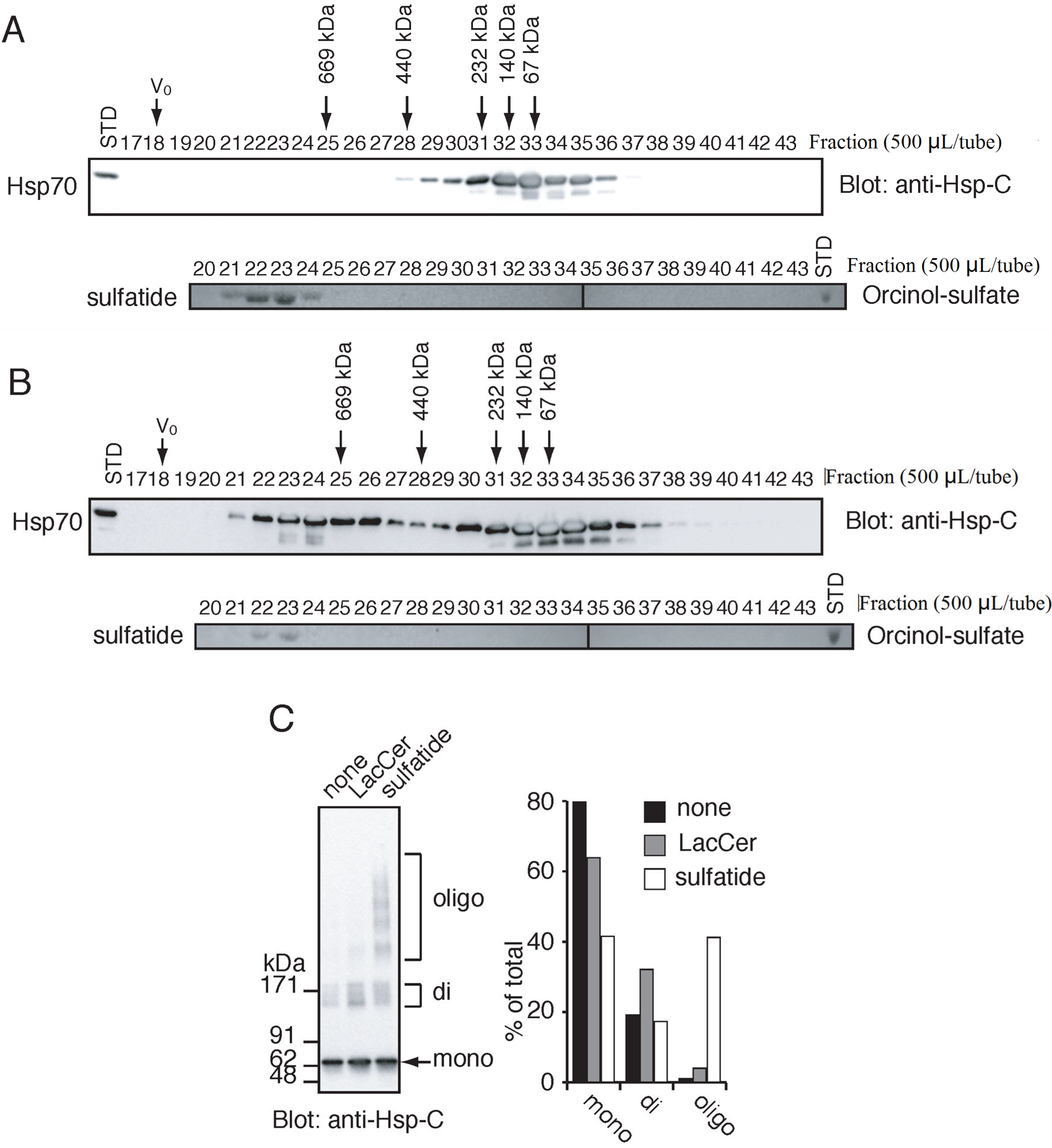
2.1. Characterization of the Purified Hsp70 and Sulfatide by Gel-Filtration Chromatography
2.2. Sulfatide Induces Higher Ordered Oligomerization of Hsp70
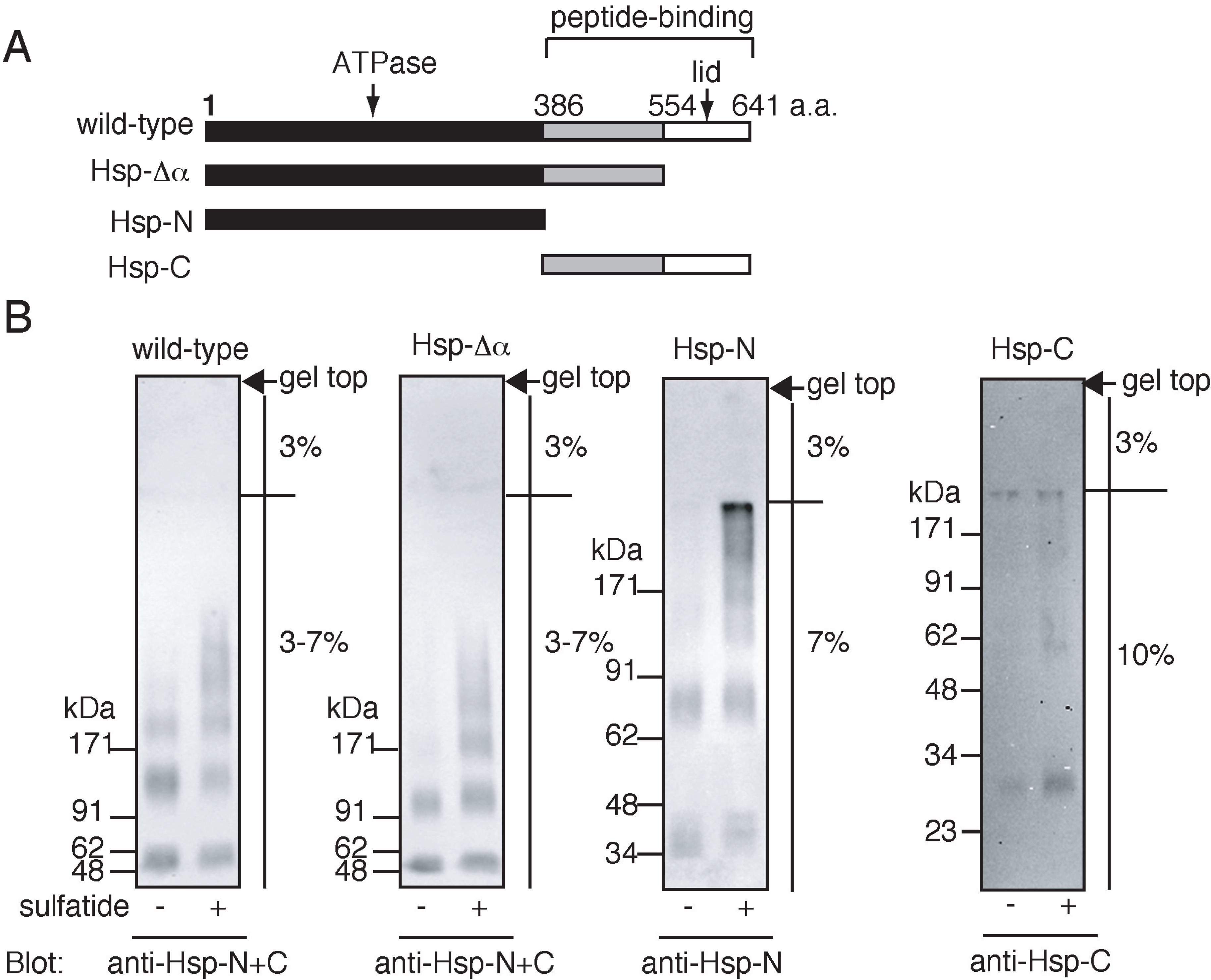
2.3. Sulfatide-Induced Oligomerization of Hsp70 through the ATPase Domain
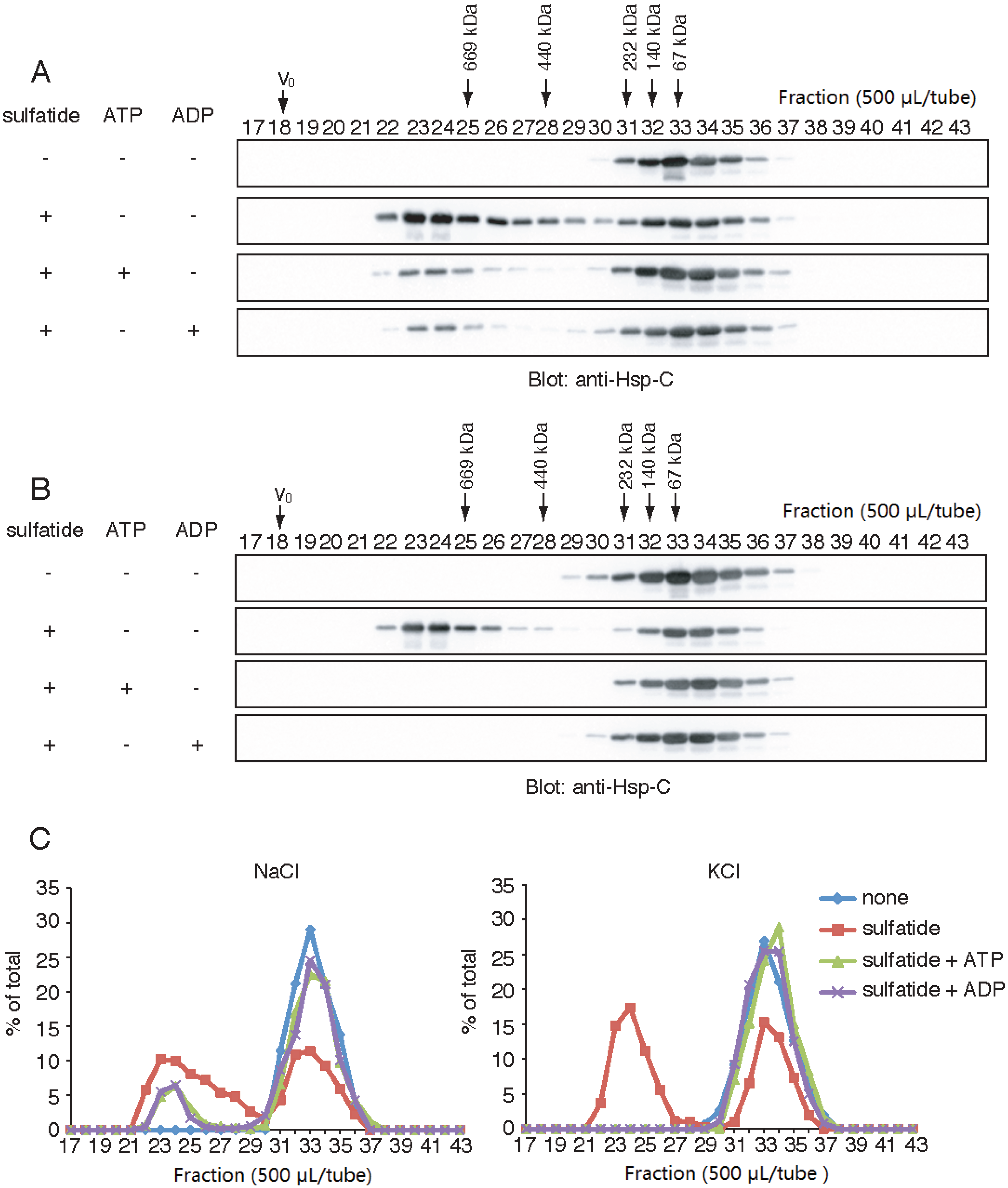
2.4. Effects of K+, Na+ and Nucleotides on the Sulfatide-Induced Formation of the HMW Hsp70
2.5. Loss of ATP-Binding Activity of the HMW Hsp70
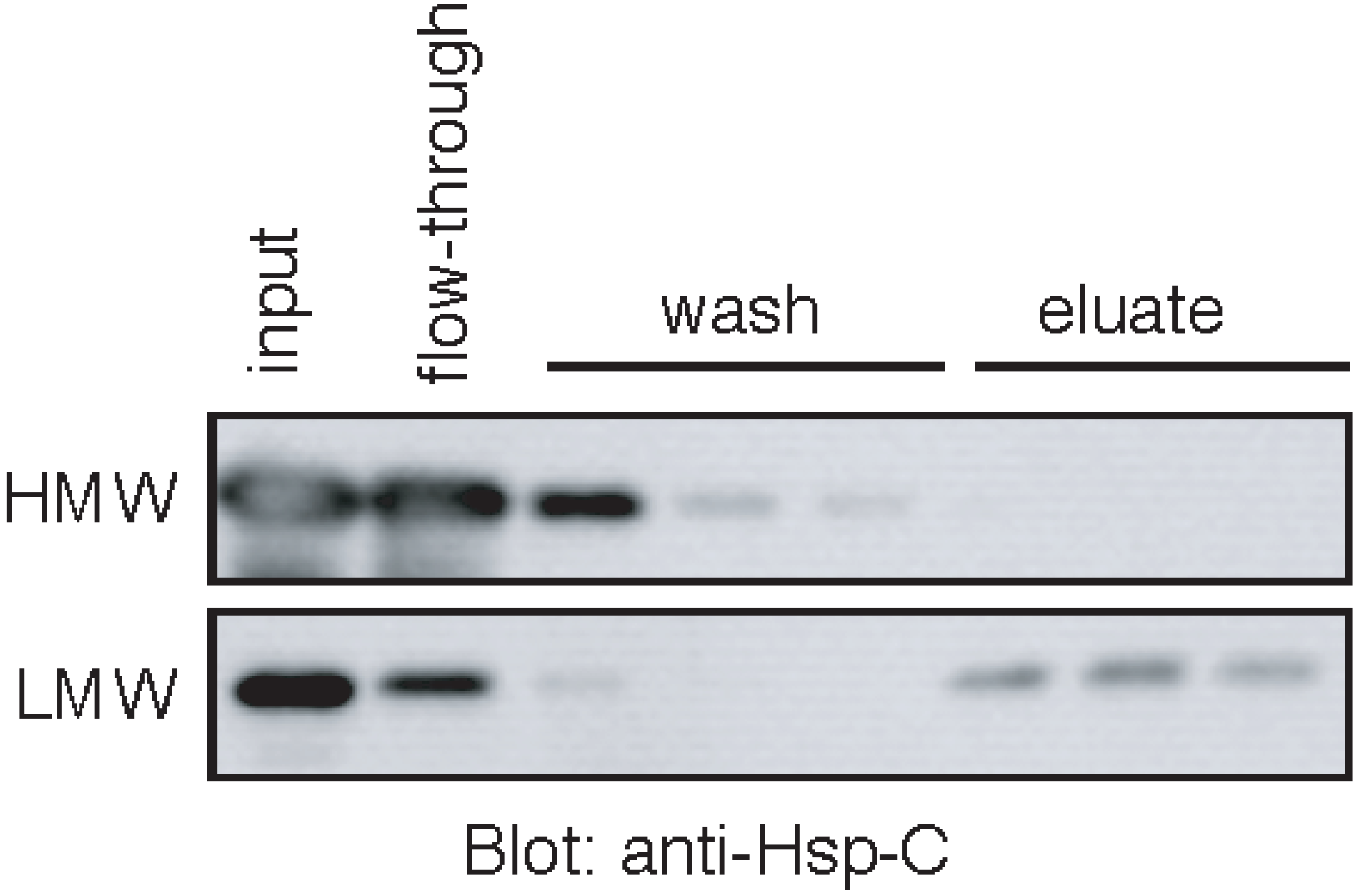
2.6. Stable Complex Formation between the HMW Hsp70 and Unfolded Protein

3. Discussion
4. Experimental Section
4.1. Materials
4.2. Protein Purification
4.3. Gel-Filtration Chromatography
4.4. Cross-Linking Experiments
4.5. ATP-Agarose Binding Assay
4.6. Interaction between Hsp70 and cd-OVA
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Hartl, F.U. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; Calderwood, S. Characterization and sequence of a mouse Hsp70 gene and its expression in mouse cell lines. Gene 1990, 87, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.D.; Aujame, L.; Shtang, S.; Moran, L.A. Structure and expression of an inducible Hsp70-encoding gene from Mus musculus. Gene 1994, 146, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.M.; DeLuca-Flaherty, C.; McKay, D.B. Three-dimensional structure of the ATPase fragment of a 70 K heat-shock cognate protein. Nature 1990, 346, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Sriram, M.; Osipiuk, J.; Freeman, B.; Morimoto, R.; Joachimiak, A. Human Hsp70 molecular chaperone binds two calcium ions within the ATPase domain. Structure 1997, 5, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Greene, L.; Eisenberg, E. Characterization of nucleotide-free uncoating ATPase and its binding to ATP, ADP, and ATP analogues. Biochemistry 1994, 33, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Wilbanks, S.M.; DeLuca-Flaherty, C.; McKay, D.B. Structural basis of the 70-kilodalton heat shock cognate protein atp hydrolytic activity. I. Kinetic analyses of active site mutants. J. Biol. Chem. 1994, 269, 12893–12898. [Google Scholar] [PubMed]
- Palleros, D.R.; Shi, L.; Reid, K.L.; Fink, A.L. Hsp70-protein complexes. Complex stability and conformation of bound substrate protein. J. Biol. Chem. 1994, 269, 13107–13114. [Google Scholar] [PubMed]
- Greene, L.E.; Zinner, R.; Naficy, S.; Eisenberg, E. Effect of nucleotide on the binding of peptides to 70-kDa heat shock protein. J. Biol. Chem. 1995, 270, 2967–2973. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Febbraio, M.A. Exosome-dependent trafficking of Hsp70: A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005, 280, 23349–23355. [Google Scholar] [CrossRef] [PubMed]
- Evdokimovskaya, Y.; Skarga, Y.; Vrublevskaya, V.; Morenkov, O. Secretion of the heat shock proteins HSP70 and HSC70 by baby hamster kidney (BHK-21) cells. Cell Biol. Int. 2010, 34, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Broquet, A.H.; Thomas, G.; Masliah, J.; Trugnan, G.; Bachelet, M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 2003, 278, 21601–21606. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Cao, M.M.; Wang, W.B.; Wang, W.; Ren, H.; Zhao, P.; Qi, Z.T. Association of heat-shock protein 70 with lipid rafts is required for japanese encephalitis virus infection in Huh7 cells. J. Gen. Virol. 2012, 93, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, J.; Faulds, D.; Eddy, E.M.; Lingwood, C.A. Members of the 70 kDa heat shock protein family specifically recognize sulfoglycolipids: Role in gamete recognition and mycoplasma-related infertility. J. Cell. Physiol. 1995, 165, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Maehashi, E.; Sato, C.; Ohta, K.; Harada, Y.; Matsuda, T.; Hirohashi, N.; Lennarz, W.J.; Kitajima, K. Identification of the sea urchin 350-kDa sperm-binding protein as a new sialic acid-binding lectin that belongs to the heat shock protein 110 family: Implication of its binding to gangliosides in sperm lipid rafts in fertilization. J. Biol. Chem. 2003, 278, 42050–42057. [Google Scholar] [CrossRef] [PubMed]
- Millar, D.G.; Garza, K.M.; Odermatt, B.; Elford, A.R.; Ono, N.; Li, Z.; Ohashi, P.S. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat. Med. 2003, 9, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Bendz, H.; Ruhland, S.C.; Pandya, M.J.; Hainzl, O.; Riegelsberger, S.; Brauchle, C.; Mayer, M.P.; Buchner, J.; Issels, R.D.; Noessner, E. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J. Biol. Chem. 2007, 282, 31688–31702. [Google Scholar] [CrossRef] [PubMed]
- Guidon, P.T., Jr.; Hightower, L.E. Purification and initial characterization of the 71-kilodalton rat heat-shock protein and its cognate as fatty acid binding proteins. Biochemistry 1986, 25, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Arispe, N.; Doh, M.; Simakova, O.; Kurganov, B.; de Maio, A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004, 18, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Arispe, N.; de Maio, A. ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J. Biol. Chem. 2000, 275, 30839–30843. [Google Scholar] [CrossRef] [PubMed]
- Law, H.; Itkonnen, O.; Lingwood, C.A. Sulfogalactolipid binding protein slip 1: A conserved function for a conserved protein. J. Cell. Physiol. 1988, 137, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Sato, C.; Kitajima, K. Complex formation of 70-kDa heat shock protein with acidic glycolipids and phospholipids. Biochem. Biophys. Res. Commun. 2007, 353, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, M.; Liebisch, G.; Schmitz, G.; Anderson, R.; Steinem, C.; de Maio, A.; Pockley, G.; Multhoff, G. Tumor-specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3. PLoS ONE 2008, 3, e1925. [Google Scholar] [CrossRef] [PubMed]
- Huesca, M.; Goodwin, A.; Bhagwansingh, A.; Hoffman, P.; Lingwood, C.A. Characterization of an acidic-pH-inducible stress protein (Hsp70), a putative sulfatide binding adhesin, from Helicobacter pylori. Infect. Immun. 1998, 66, 4061–4067. [Google Scholar] [PubMed]
- Hartmann, E.; Lingwood, C.A.; Reidl, J. Heat-inducible surface stress protein (Hsp70) mediates sulfatide recognition of the respiratory pathogen haemophilus influenzae. Infect. Immun. 2001, 69, 3438–3441. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, D.; Mylvaganam, M.; Whetstone, H.; Hartmann, E.; Lennarz, W.; Wyrick, P.B.; Raulston, J.; Han, H.; Hoffman, P.; Lingwood, C.A. Hsp70s contain a specific sulfogalactolipid binding site. Differential aglycone influence on sulfogalactosyl ceramide binding by recombinant prokaryotic and eukaryotic Hsp70 family members. Biochemistry 2001, 40, 3572–3582. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, D.; Lingwood, C. The atpase domain of hsp70 possesses a unique binding specificity for 3'-sulfogalactolipids. J. Biol. Chem. 2001, 276, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, D.; Mylvaganam, M.; Tanahashi, E.; Ito, H.; Ishida, H.; Kiso, M.; Lingwood, C. The aglycone of sulfogalactolipids can alter the sulfate ester substitution position required for Hsc70 recognition. Carbohydr. Res. 2001, 335, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Whetstone, H.; Lingwood, C. 3'-sulfogalactolipid binding specifically inhibits Hsp70 ATPase activity in vitro. Biochemistry 2003, 42, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Aprile, F.A.; Dhulesia, A.; Stengel, F.; Roodveldt, C.; Benesch, J.L.; Tortora, P.; Robinson, C.V.; Salvatella, X.; Dobson, C.M.; Cremades, N. Hsp70 oligomerization is mediated by an interaction between the interdomain linker and the substrate-binding domain. PLoS ONE 2013, 8, e67961. [Google Scholar] [CrossRef] [PubMed]
- Angelidis, C.E.; Lazaridis, I.; Pagoulatos, G.N. Aggregation of Hsp70 and Hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur. J. Biochem. FEBS 1999, 259, 505–512. [Google Scholar] [CrossRef]
- Benaroudj, N.; Triniolles, F.; Ladjimi, M.M. Effect of nucleotides, peptides, and unfolded proteins on the self-association of the molecular chaperone Hsc70. J. Biol. Chem. 1996, 271, 18471–18476. [Google Scholar] [CrossRef] [PubMed]
- Breloer, M.; Marti, T.; Fleischer, B.; von Bonin, A. Isolation of processed, H-2Kb-binding ovalbumin-derived peptides associated with the stress proteins Hsp70 and Gp96. Eur. J. Immunol. 1998, 28, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Sato, C.; Matsuda, T.; Toriyama, M.; Lennarz, W.J.; Kitajima, K. Isolation and characterization of low density detergent-insoluble membrane (LD-DIM) fraction from sea urchin sperm. Biochem. Biophys. Res. Commun. 1999, 258, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Moyano, A.L.; Li, G.; Lopez-Rosas, A.; Mansson, J.E.; van Breemen, R.B.; Givogri, M.I. Distribution of C16:0, C18:0, C24:1, and C24:0 sulfatides in central nervous system lipid rafts by quantitative ultra-high-pressure liquid chromatography tandem mass spectrometry. Anal. Biochem. 2014, 467, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bork, P.; Sander, C.; Valencia, A. An atpase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and Hsp70 heat shock proteins. Proc. Natl. Acad. Sci. USA 1992, 89, 7290–7294. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.S., Jr.; Steitz, T.A. Structure of a complex between yeast hexokinase a and glucose. II. Detailed comparisons of conformation and active site configuration with the native hexokinase B monomer and dimer. J. Mol. Biol. 1980, 140, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.S., Jr.; Steitz, T.A. Structure of a complex between yeast hexokinase a and glucose. I. Structure determination and refinement at 3.5 Å resolution. J. Mol. Biol. 1980, 140, 183–209. [Google Scholar] [CrossRef] [PubMed]
- Wilbanks, S.M.; McKay, D.B. How potassium affects the activity of the molecular chaperone Hsc70. II. Potassium binds specifically in the ATPase active site. J. Biol. Chem. 1995, 270, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.C.; McKay, D.B. How potassium affects the activity of the molecular chaperone Hsc70. I. Potassium is required for optimal ATPase activity. J. Biol. Chem. 1995, 270, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Imamura, H.; Suzuki, R.; Aizaki, H.; Watanabe, T.; Wakita, T.; Suzuki, T. Visualization and measurement of atp levels in living cells replicating hepatitis C virus genome rna. PLoS Pathog. 2012, 8, e1002561. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Garenaux, E.; Nagatsuka, T.; Uzawa, H.; Nishida, Y.; Sato, C.; Kitajima, K. Interaction of 70-kDa heat shock protein with glycosaminoglycans and acidic glycopolymers. Biochem. Biophys. Res. Commun. 2014, 453, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L. The quantitative estimation of cerebrosides in nervous tissue. J. Neurochem. 1956, 1, 42–53. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harada, Y.; Sato, C.; Kitajima, K. Sulfatide-Hsp70 Interaction Promotes Hsp70 Clustering and Stabilizes Binding to Unfolded Protein. Biomolecules 2015, 5, 958-973. https://doi.org/10.3390/biom5020958
Harada Y, Sato C, Kitajima K. Sulfatide-Hsp70 Interaction Promotes Hsp70 Clustering and Stabilizes Binding to Unfolded Protein. Biomolecules. 2015; 5(2):958-973. https://doi.org/10.3390/biom5020958
Chicago/Turabian StyleHarada, Yoichiro, Chihiro Sato, and Ken Kitajima. 2015. "Sulfatide-Hsp70 Interaction Promotes Hsp70 Clustering and Stabilizes Binding to Unfolded Protein" Biomolecules 5, no. 2: 958-973. https://doi.org/10.3390/biom5020958





