Impact of Vitamin D3 Deficiency on Phosphatidylcholine-/Ethanolamine, Plasmalogen-, Lyso-Phosphatidylcholine-/Ethanolamine, Carnitine- and Triacyl Glyceride-Homeostasis in Neuroblastoma Cells and Murine Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Standards
2.2. Cell Culture and Calcitriol-Treatment
2.3. Animal Experiments
2.4. Sample Preparation
2.5. Targeted Shotgun Mass Spectrometry
2.6. Gene Expression Analysis
2.7. Analysis of Oxygen Consumption
2.8. Data and Statistical Analysis
3. Results
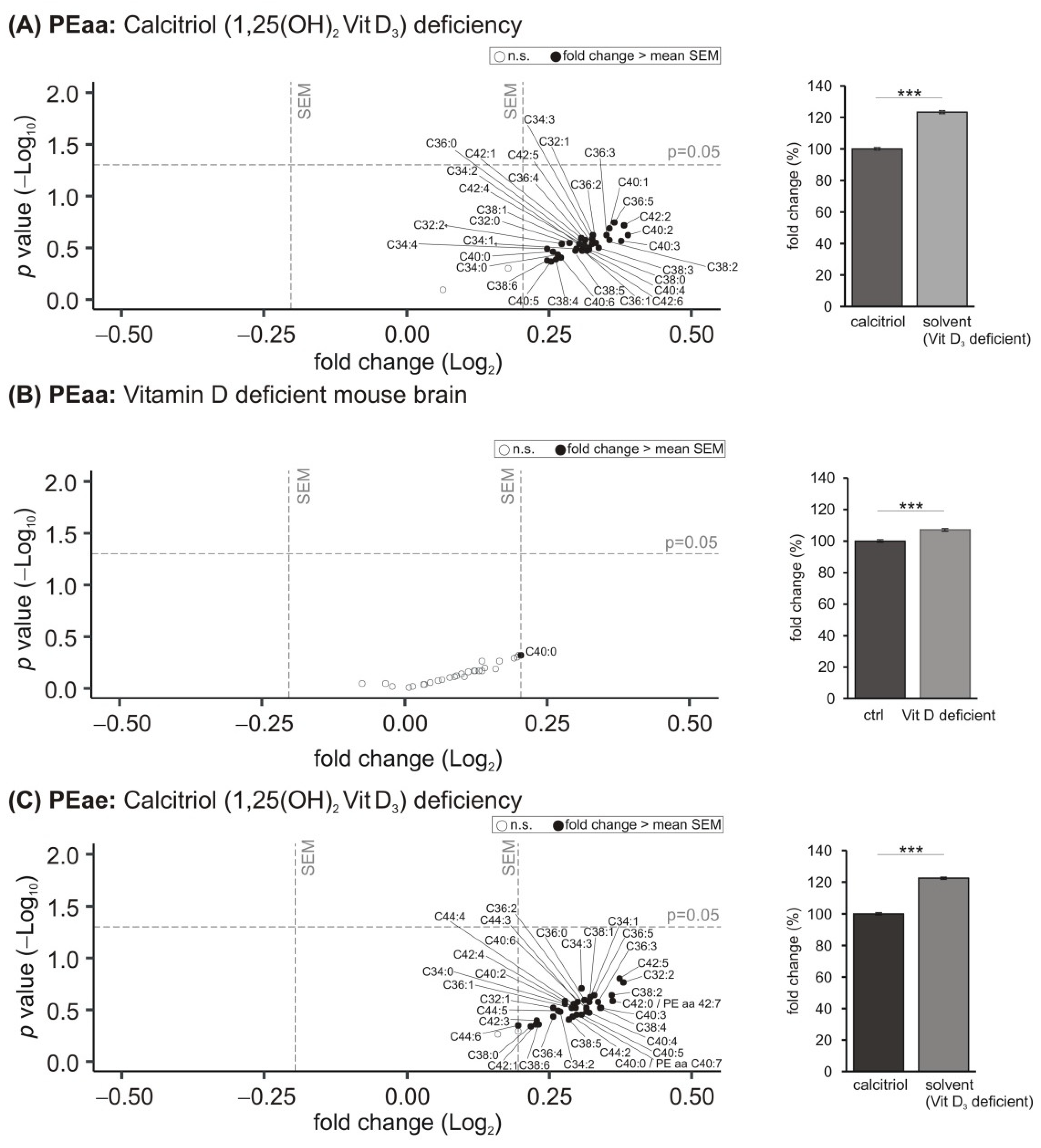
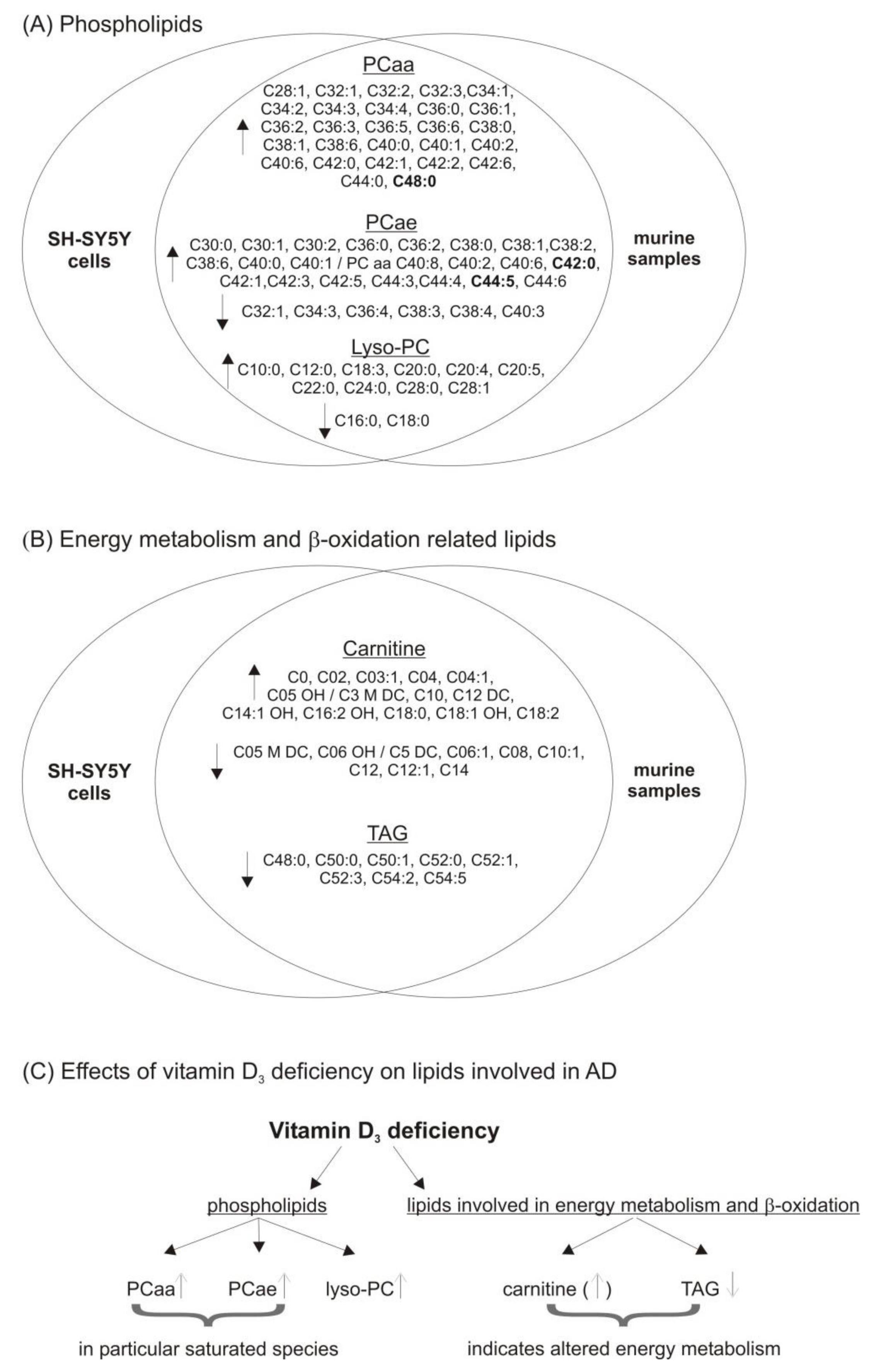
3.1. Phosphatidylcholine (PCaa) Species
3.2. Phosphatidylcholine Plasmalogens (PCae) Species
3.3. Lyso-Phosphatidylcholine (Lyso-PC) Species
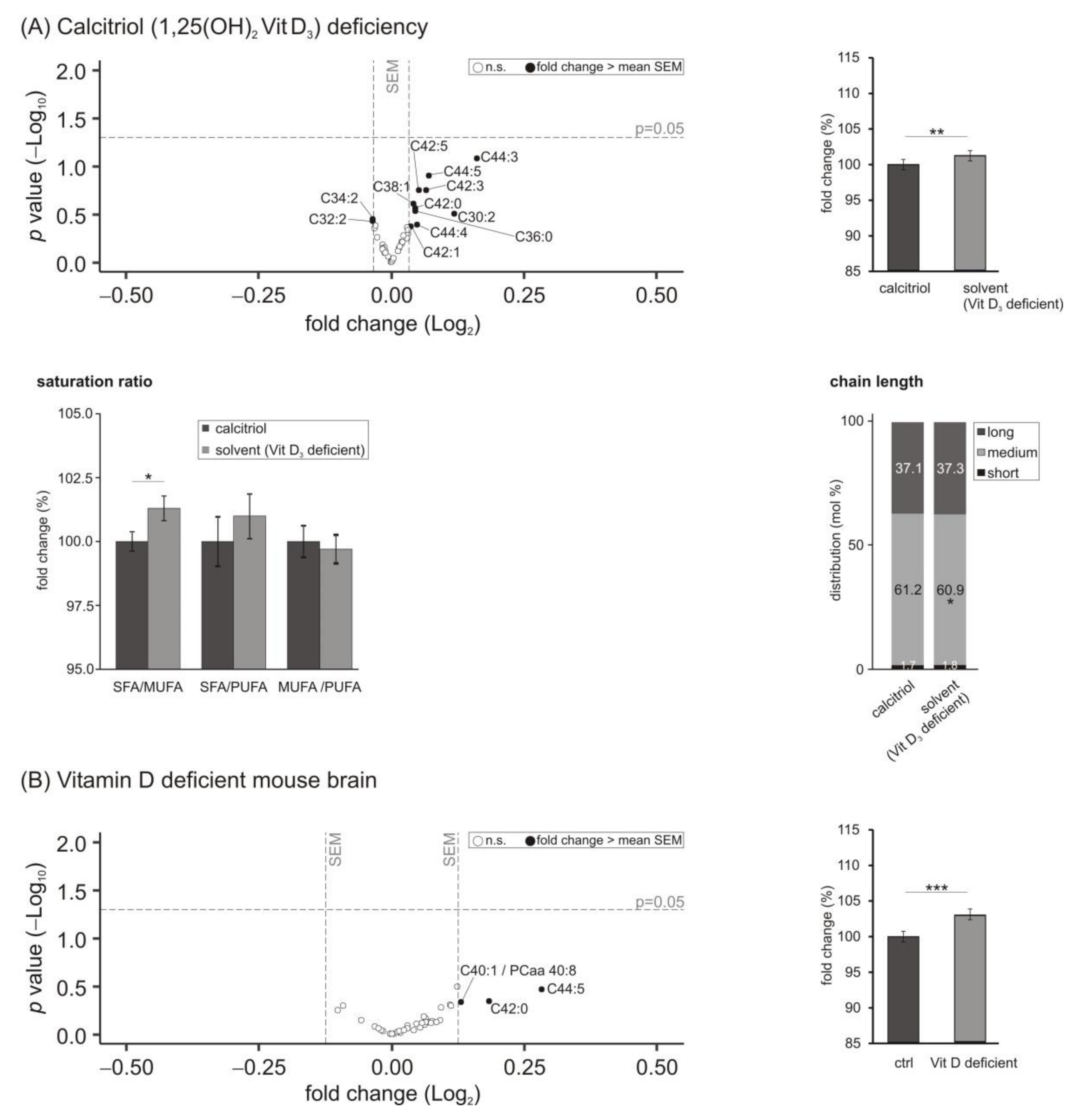
3.4. Phosphatidylethanolamine (PE) Species
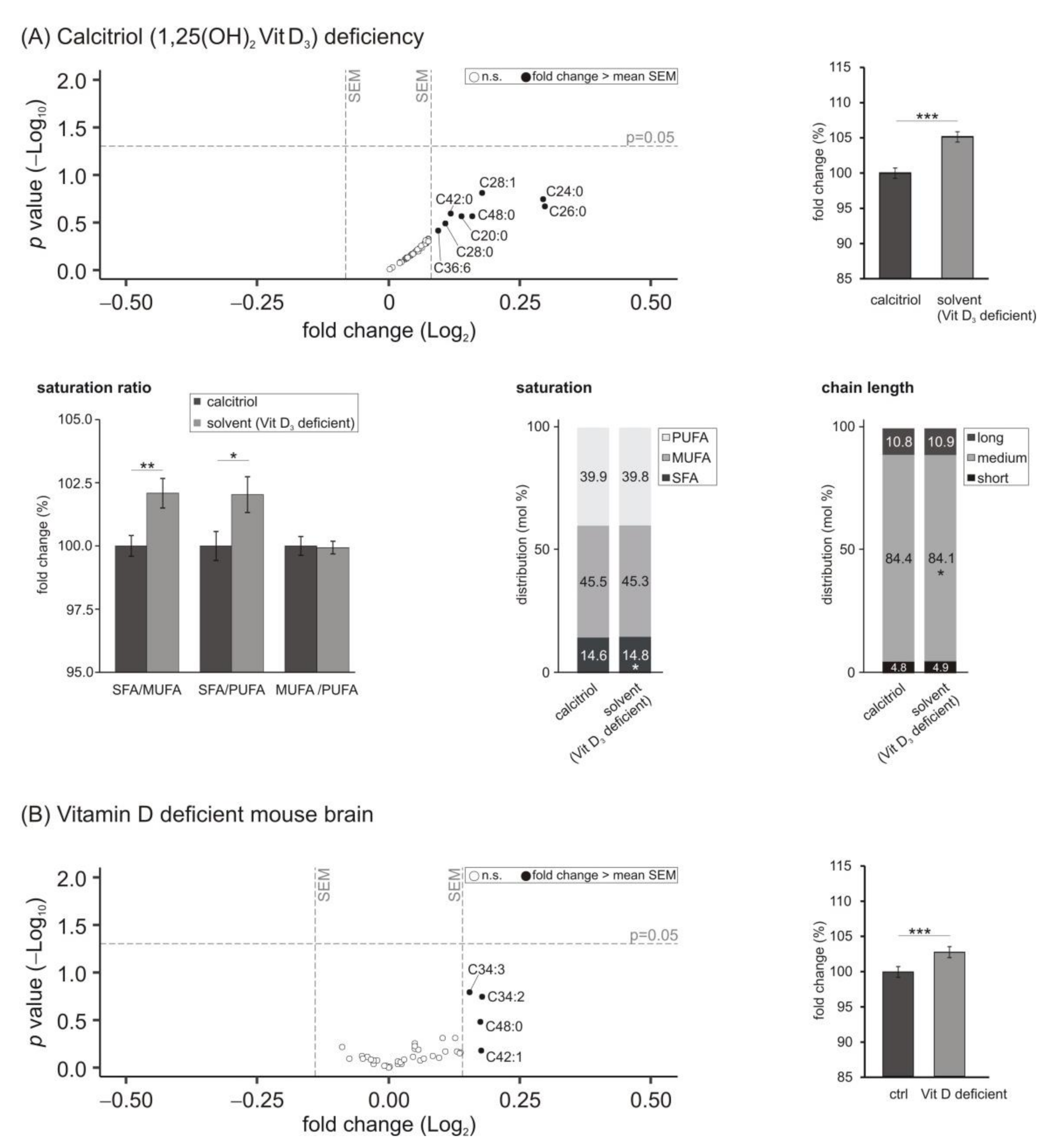
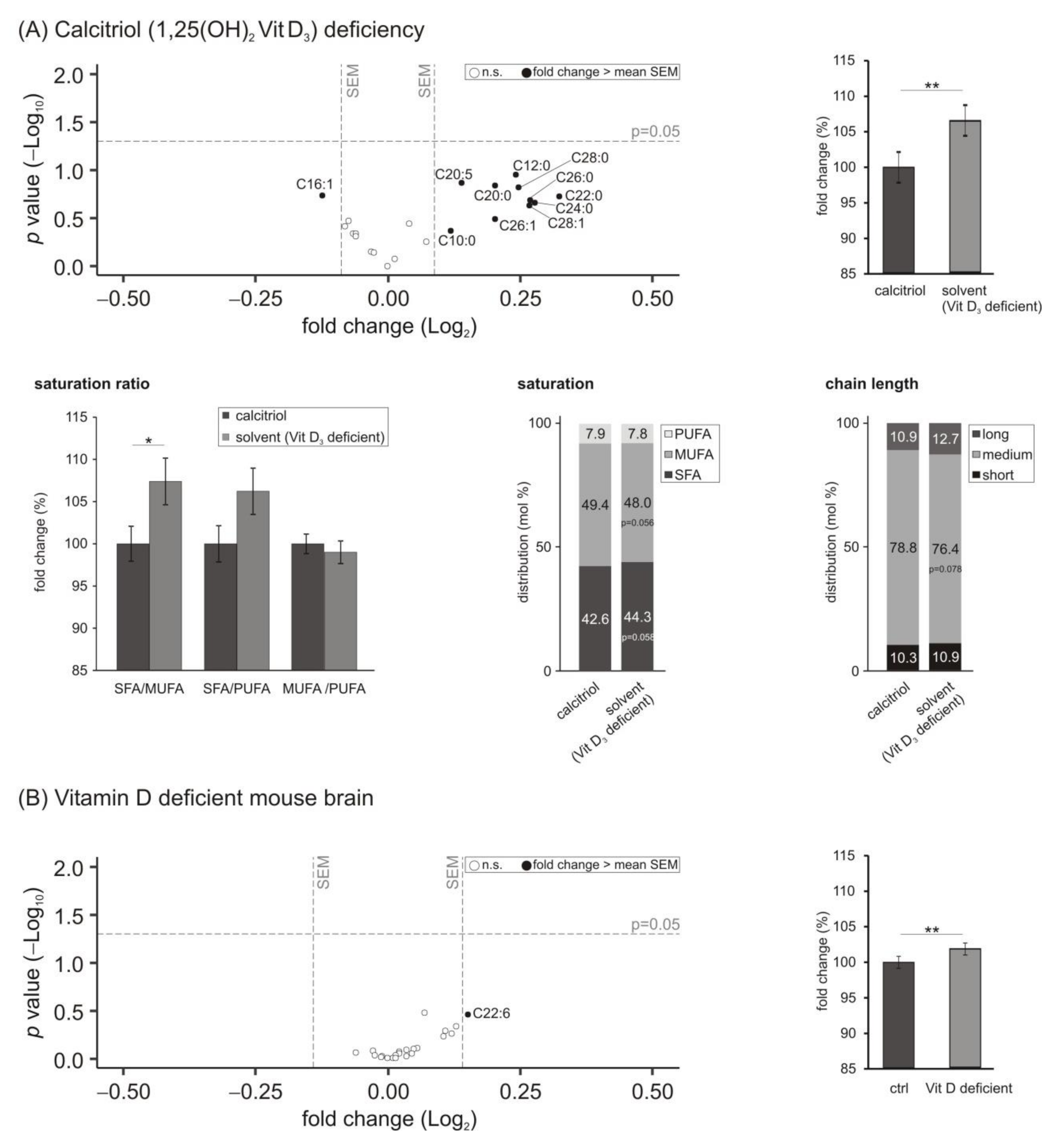
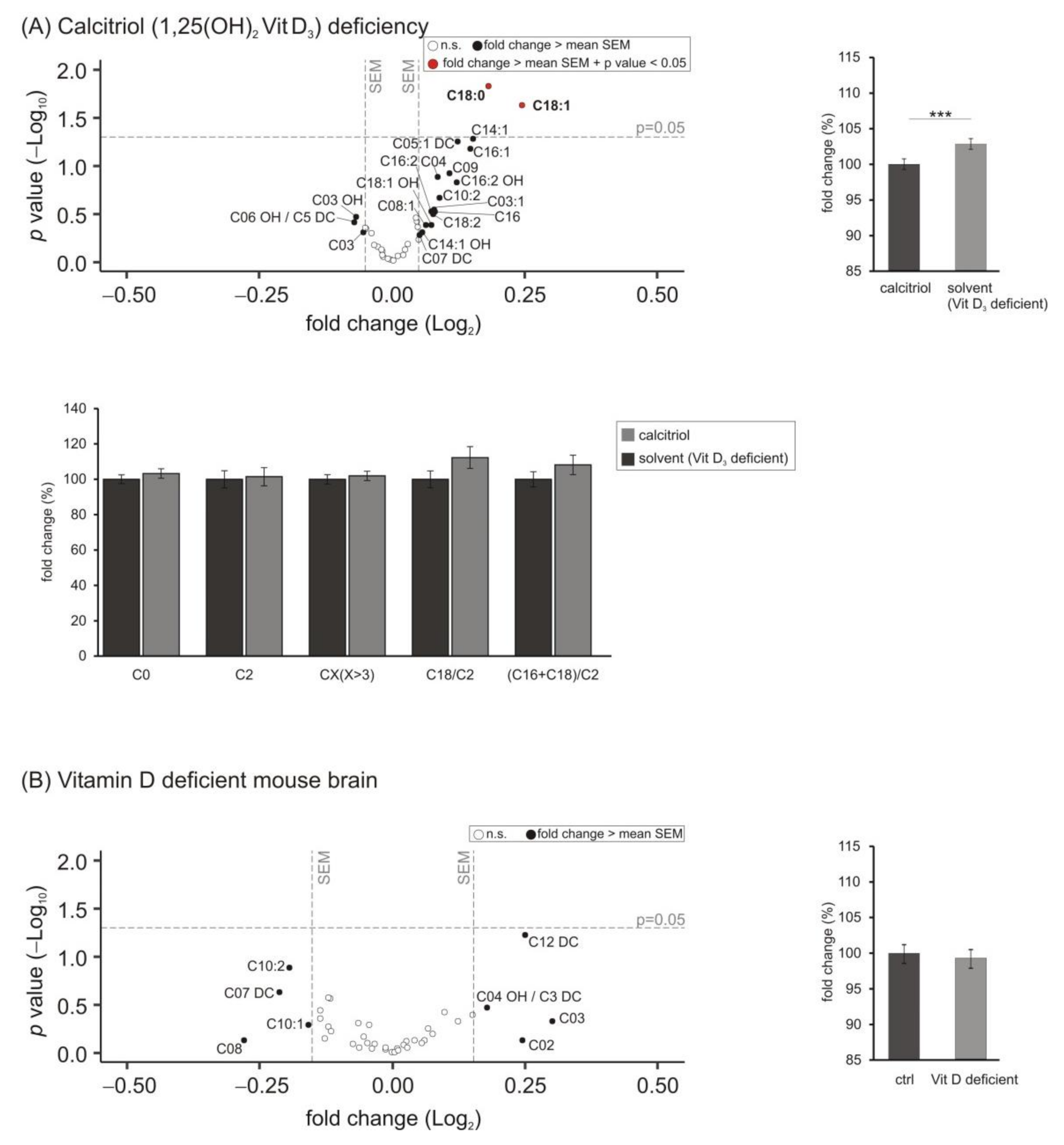
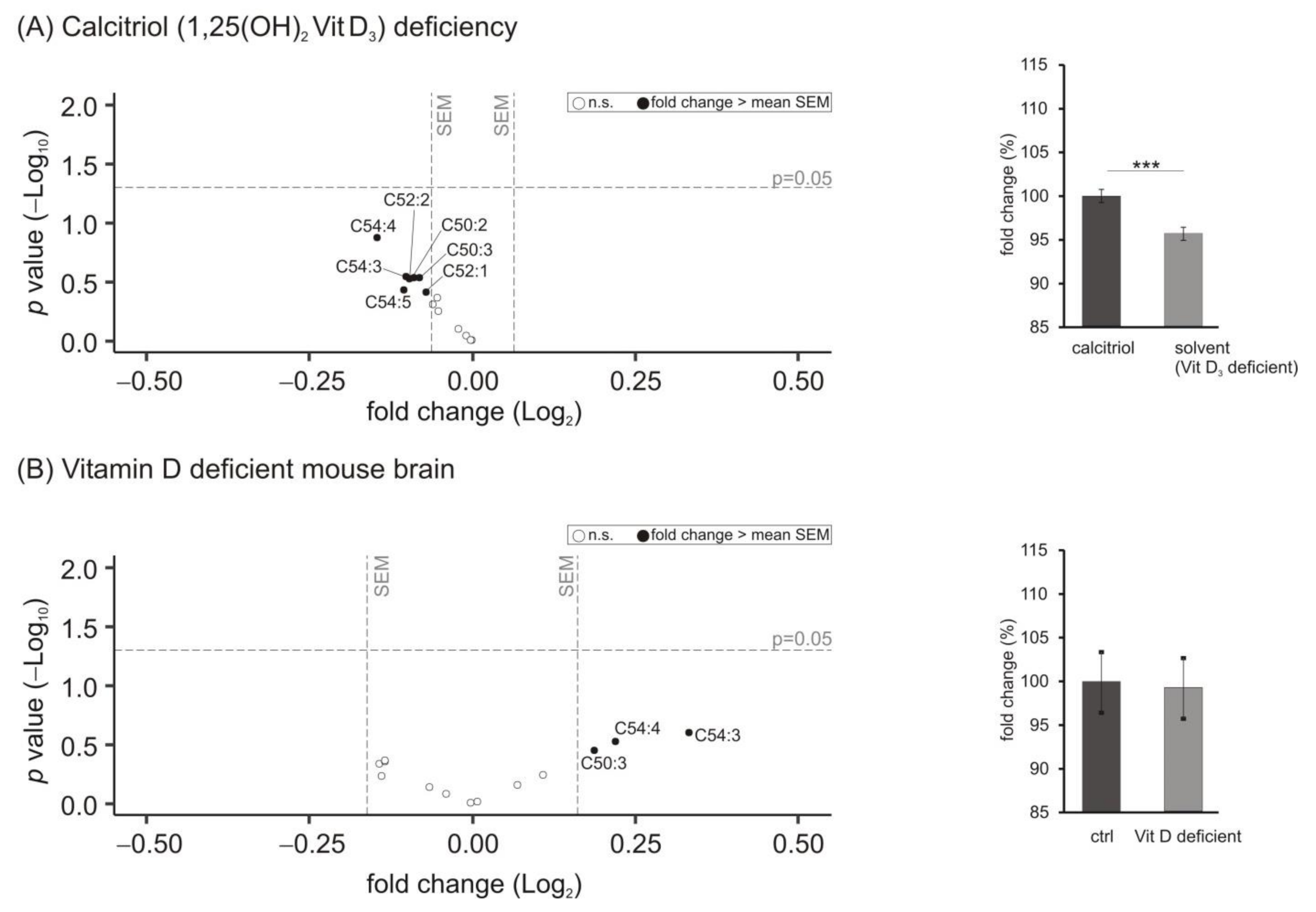
3.5. Lipid Species Involved in Cellular Energy Metabolism and β-Oxidation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin, D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Kweder, H.; Eidi, H. Vitamin D deficiency in elderly: Risk factors and drugs impact on Vitamin D status. Avicenna J. Med. 2018, 8, 139–146. [Google Scholar] [CrossRef]
- Webb, A.R.; DeCosta, B.R.; Holick, M.F. Sunlight regulates the cutaneous production of Vitamin D3 by causing its photodegradation. J. Clin. Endocrinol. Metab. 1989, 68, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D and bone health. J. Nutr. 1996, 126, 1159S–1164S. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, T.; Lim, K.; Thadhani, R.; Manson, J.E. Vitamin D and atherosclerotic cardiovascular disease. J. Clin. Endocrinol. Metab. 2019, 104, 4033–4050. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Mondal, S.A.; Choudhuri, S.; Maisnam, I.; Hasanoor Reza, A.H.; Bhattacharya, B.; Chowdhury, S.; Mukhopadhyay, S. Vitamin-d supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: An open label randomized prospective study from eastern india. Diabetes Res. Clin. Pract. 2014, 103, e18–e23. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Lauer, A.A.; Grosgen, S.; Thiel, A.; Lehmann, J.; Winkler, J.; Janitschke, D.; Herr, C.; Beisswenger, C.; Bals, R.; et al. Profiling of alzheimer’s disease related genes in mild to moderate Vitamin D hypovitaminosis. J. Nutr. Biochem. 2019, 67, 123–137. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and chronic diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Lauer, A.A.; Janitschke, D.; Hartmann, T.; Grimm, H.S.; Grimm, M.O. The effects of Vitamin D deficiency on neurodegenerative diseases, Vitamin D deficiency. In Vitamin D Deficiency; Fedotova, J., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Thompson, V.W.; Deluca, H.F. Vitamin D and phospholipid metabolism. J. Biol. Chem. 1964, 239, 984–989. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Alvarez, U.; Avioli, L.V.; Hruska, K.A. Effect of 1,25-dihydroxyVitamin D3 on phospholipid composition of rat renal brush border membrane. Am. J. Physiol. 1985, 249, F117–F123. [Google Scholar] [CrossRef]
- Alisio, A.; Canas, F.; de Bronia, D.H.; Pereira, R.; Tolosa de Talamoni, N. Effect of Vitamin D deficiency on lipid composition and calcium transport in basolateral membrane vesicles from chick intestine. Biochem. Mol. Biol. Int. 1997, 42, 339–347. [Google Scholar] [CrossRef]
- Martinez-Sena, T.; Soluyanova, P.; Guzman, C.; Valdivielso, J.M.; Castell, J.V.; Jover, R. The Vitamin D receptor regulates glycerolipid and phospholipid metabolism in human hepatocytes. Biomolecules 2020, 10, 493. [Google Scholar] [CrossRef] [Green Version]
- Jahn, D.; Dorbath, D.; Schilling, A.K.; Gildein, L.; Meier, C.; Vuille-Dit-Bille, R.N.; Schmitt, J.; Kraus, D.; Fleet, J.C.; Hermanns, H.M.; et al. Intestinal Vitamin D receptor modulates lipid metabolism, adipose tissue inflammation and liver steatosis in obese mice. Biochim. Biophys. Acta Mol. Basis. Dis. 2019, 1865, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, I.; Lu, R.; Zhang, Y.; Zhang, J.; Dai, Y.; Xia, Y.; Sun, J. Vitamin D receptor promotes healthy microbial metabolites and microbiome. Sci. Rep. 2020, 10, 7340. [Google Scholar] [CrossRef]
- Challoumas, D. Vitamin D supplementation and lipid profile: What does the best available evidence show? Atherosclerosis 2014, 235, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Si, S.; Liu, J.; Wang, Z.; Jia, H.; Feng, K.; Sun, L.; Song, S.J. The associations of serum lipids with Vitamin D status. PLoS ONE 2016, 11, e0165157. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Thiel, A.; Lauer, A.A.; Winkler, J.; Lehmann, J.; Regner, L.; Nelke, C.; Janitschke, D.; Benoist, C.; Streidenberger, O.; et al. Vitamin D and its analogues decrease amyloid-beta (abeta) formation and increase abeta-degradation. Int. J. Mol. Sci. 2017, 18, 2764. [Google Scholar] [CrossRef] [Green Version]
- Landfield, P.W.; Cadwallader-Neal, L. Long-term treatment with calcitriol (1,25(oh)2 vit d3) retards a biomarker of hippocampal aging in rats. Neurobiol. Aging 1998, 19, 469–477. [Google Scholar] [CrossRef]
- Lang, F.; Ma, K.; Leibrock, C.B. 1,25(oh)2d3 in brain function and neuropsychiatric disease. Neurosignals 2019, 27, 40–49. [Google Scholar] [PubMed]
- Kim, Y.C.; Lee, J.; Lee, D.W.; Jeong, B.H. Large-scale lipidomic profiling identifies novel potential biomarkers for prion diseases and highlights lipid raft-related pathways. Vet. Res. 2021, 52, 105. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The role of lipids in parkinson’s disease. Cells 2019, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Toshniwal, P.K.; Zarling, E.J. Evidence for increased lipid peroxidation in multiple sclerosis. Neurochem. Res. 1992, 17, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.L.; Grimsgaard, A. Multiple sclerosis: The lipid relationship. Am. J. Clin. Nutr. 1988, 48, 1387–1393. [Google Scholar] [CrossRef] [Green Version]
- Beghi, E.; Pupillo, E.; Bonito, V.; Buzzi, P.; Caponnetto, C.; Chio, A.; Corbo, M.; Giannini, F.; Inghilleri, M.; Bella, V.L.; et al. Randomized double-blind placebo-controlled trial of acetyl-l-carnitine for als. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Green, P.; John Mann, J.; Rapoport, S.I.; Sublette, M.E. Pathways of polyunsaturated fatty acid utilization: Implications for brain function in neuropsychiatric health and disease. Brain Res. 2015, 1597, 220–246. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef] [Green Version]
- Chiurchiu, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haffner, S.M. Management of dyslipidemia in adults with diabetes. Diabetes Care 1998, 21, 160–178. [Google Scholar] [CrossRef]
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and lipids in cardiovascular disease: From mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef]
- Pei, K.; Gui, T.; Kan, D.; Feng, H.; Jin, Y.; Yang, Y.; Zhang, Q.; Du, Z.; Gai, Z.; Wu, J.; et al. An overview of lipid metabolism and nonalcoholic fatty liver disease. BioMed Res. Int. 2020, 2020, 4020249. [Google Scholar] [CrossRef]
- Saiki, S.; Hatano, T.; Fujimaki, M.; Ishikawa, K.I.; Mori, A.; Oji, Y.; Okuzumi, A.; Fukuhara, T.; Koinuma, T.; Imamichi, Y.; et al. Decreased long-chain acylcarnitines from insufficient beta-oxidation as potential early diagnostic markers for parkinson’s disease. Sci. Rep. 2017, 7, 7328. [Google Scholar] [CrossRef] [PubMed]
- Jakubec, M.; Barias, E.; Kryuchkov, F.; Hjornevik, L.V.; Halskau, O. Fast and quantitative phospholipidomic analysis of sh-sy5y neuroblastoma cell cultures using liquid chromatography-tandem mass spectrometry and (31)p nuclear magnetic resonance. ACS Omega 2019, 4, 21596–21603. [Google Scholar] [CrossRef] [Green Version]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In vitro models of neurodegenerative diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Lindstrom, T.; Kagedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of sh-sy5y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The sh-sy5y cell line in parkinson’s disease research: A systematic review. Mol. Neurodegener 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, M.O.; Lehmann, J.; Mett, J.; Zimmer, V.C.; Grosgen, S.; Stahlmann, C.P.; Hundsdorfer, B.; Haupenthal, V.J.; Rothhaar, T.L.; Herr, C.; et al. Impact of Vitamin D on amyloid precursor protein processing and amyloid-beta peptide degradation in alzheimer’s disease. Neurodegener Dis. 2014, 13, 75–81. [Google Scholar] [CrossRef]
- Annweiler, C.; Souberbielle, J.C.; Schott, A.M.; de Decker, L.; Berrut, G.; Beauchet, O. Vitamin D in the elderly: 5 points to remember. Geriatr. Psychol. Neuropsychiatr. Vieil. 2011, 9, 259–267. [Google Scholar] [CrossRef]
- Buell, J.S.; Dawson-Hughes, B.; Scott, T.M.; Weiner, D.E.; Dallal, G.E.; Qui, W.Q.; Bergethon, P.; Rosenberg, I.H.; Folstein, M.F.; Patz, S.; et al. 25-hydroxyVitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 2010, 74, 18–26. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Grimm, M.O.; Grosgen, S.; Riemenschneider, M.; Tanila, H.; Grimm, H.S.; Hartmann, T. From brain to food: Analysis of phosphatidylcholins, lyso-phosphatidylcholins and phosphatidylcholin-plasmalogens derivates in alzheimer’s disease human post mortem brains and mice model via mass spectrometry. J. Chromatogr. A 2011, 1218, 7713–7722. [Google Scholar] [CrossRef]
- Lauer, A.A.; Janitschke, D.; Dos Santos Guilherme, M.; Nguyen, V.T.T.; Bachmann, C.M.; Qiao, S.; Schrul, B.; Boehm, U.; Grimm, H.S.; Hartmann, T.; et al. Shotgun lipidomics of liver and brain tissue of alzheimer’s disease model mice treated with acitretin. Sci. Rep. 2021, 11, 15301. [Google Scholar] [CrossRef]
- Kurokin, I.; Lauer, A.A.; Janitschke, D.; Winkler, J.; Theiss, E.L.; Griebsch, L.V.; Pilz, S.M.; Matschke, V.; van der Laan, M.; Grimm, H.S.; et al. Targeted lipidomics of mitochondria in a cellular alzheimer’s disease model. Biomedicines 2021, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, H.; Kittlaus, S.; Kempe, G.; Alder, L. Reduction of matrix effects in liquid chromatography-electrospray ionization-mass spectrometry by dilution of the sample extracts: How much dilution is needed? Anal. Chem. 2012, 84, 1474–1482. [Google Scholar] [CrossRef]
- Panuwet, P.; Hunter, R.E., Jr.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological matrix effects in quantitative tandem mass spectrometry-based analytical methods: Advancing biomonitoring. Crit. Rev. Anal. Chem. 2016, 46, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Li, M.; Dong, Y.; Zhang, X.; Liu, X.; Chen, Z.; Zhu, Y.; Wang, H.; Liu, X.; Zhu, J.; et al. 1alpha,25-dihydroxyVitamin D3 up-regulates il-34 expression in sh-sy5y neural cells. Innate Immun. 2017, 23, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Sastry, P.S. Lipids of nervous tissue: Composition and metabolism. Prog. Lipid Res. 1985, 24, 69–176. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [Green Version]
- Dyrks, T.; Weidemann, A.; Multhaup, G.; Salbaum, J.M.; Lemaire, H.G.; Kang, J.; Muller-Hill, B.; Masters, C.L.; Beyreuther, K. Identification, transmembrane orientation and biogenesis of the amyloid a4 precursor of alzheimer’s disease. EMBO J. 1988, 7, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Haass, C. Take five--bace and the gamma-secretase quartet conduct alzheimer’s amyloid beta-peptide generation. EMBO J. 2004, 23, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Kaether, C.; Thinakaran, G.; Sisodia, S. Trafficking and proteolytic processing of app. Cold Spring Harb. Perspect. Med. 2012, 2, a006270. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [Green Version]
- Hsu, F.F. Mass spectrometry-based shotgun lipidomics-A critical review from the technical point of view. Anal. Bioanal. Chem. 2018, 410, 6387–6409. [Google Scholar] [CrossRef]
- Buszewski, B.; Walczak, J.; Zuvela, P.; Liu, J.J. Non-target analysis of phospholipid and sphingolipid species in egg yolk using liquid chromatography/triple quadrupole tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 179–186. [Google Scholar] [CrossRef]
- Shui, G.; Stebbins, J.W.; Lam, B.D.; Cheong, W.F.; Lam, S.M.; Gregoire, F.; Kusonoki, J.; Wenk, M.R. Comparative plasma lipidome between human and cynomolgus monkey: Are plasma polar lipids good biomarkers for diabetic monkeys? PLoS ONE 2011, 6, e19731. [Google Scholar] [CrossRef] [PubMed]
- Zukunft, S.; Prehn, C.; Rohring, C.; Moller, G.; Hrabe de Angelis, M.; Adamski, J.; Tokarz, J. High-throughput extraction and quantification method for targeted metabolomics in murine tissues. Metabolomics 2018, 14, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiley, L.; Sen, A.; Heaton, J.; Proitsi, P.; Garcia-Gomez, D.; Leung, R.; Smith, N.; Thambisetty, M.; Kloszewska, I.; Mecocci, P.; et al. Evidence of altered phosphatidylcholine metabolism in alzheimer’s disease. Neurobiol. Aging 2014, 35, 271–278. [Google Scholar] [CrossRef]
- Blasko, I.; Defrancesco, M.; Oberacher, H.; Loacker, L.; Kemmler, G.; Marksteiner, J.; Humpel, C. Plasma phosphatidylcholines and vitamin b12/folate levels are possible prognostic biomarkers for progression of alzheimer’s disease. Exp. Gerontol. 2021, 147, 111264. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Derm. 2020, 29, 876–884. [Google Scholar] [CrossRef]
- Nandi, A.; Wadhwani, N.; Joshi, S.R. Vitamin D deficiency influences fatty acid metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, G.Y.; Eckert, G.P.; Lee, J.C. Cellular membrane fluidity in amyloid precursor protein processing. Mol. Neurobiol. 2014, 50, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C. Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem. Int. 2011, 58, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.C.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Schengrund, C.L. Lipid rafts: Keys to neurodegeneration. Brain Res. Bull. 2010, 82, 7–17. [Google Scholar] [CrossRef]
- El Gaamouch, F.; Jing, P.; Xia, J.; Cai, D. Alzheimer’s disease risk genes and lipid regulators. J. Alzheimer’s Dis. 2016, 53, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Kawarabayashi, T.; Shoji, M.; Younkin, L.H.; Wen-Lang, L.; Dickson, D.W.; Murakami, T.; Matsubara, E.; Abe, K.; Ashe, K.H.; Younkin, S.G. Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein e and phosphorylated tau accumulation in the tg2576 mouse model of alzheimer’s disease. J. Neurosci. 2004, 24, 3801–3809. [Google Scholar] [CrossRef]
- Okada, T.; Ikeda, K.; Wakabayashi, M.; Ogawa, M.; Matsuzaki, K. Formation of toxic abeta(1-40) fibrils on gm1 ganglioside-containing membranes mimicking lipid rafts: Polymorphisms in abeta(1-40) fibrils. J. Mol. Biol. 2008, 382, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, M.; Katafuchi, T.; Mawatari, S.; Noda, M.; Miake, K.; Sugiyama, M.; Fujino, T. Anti-inflammatory/anti-amyloidogenic effects of plasmalogens in lipopolysaccharide-induced neuroinflammation in adult mice. J. Neuroinflamm. 2012, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An updated review of lysophosphatidylcholine metabolism in human diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [Green Version]
- Zahednasab, H.; Balood, M.; Harirchian, M.H.; Mesbah-Namin, S.A.; Rahimian, N.; Siroos, B. Increased autotaxin activity in multiple sclerosis. J. Neuroimmunol. 2014, 273, 120–123. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Michikawa, M.; Kim, S.U.; Nagai, A. Lysophosphatidylcholine increases the neurotoxicity of alzheimer’s amyloid beta1-42 peptide: Role of oligomer formation. Neuroscience 2015, 292, 159–169. [Google Scholar] [CrossRef]
- Bergau, N.; Maul, S.; Rujescu, D.; Simm, A.; Navarrete Santos, A. Reduction of glycolysis intermediate concentrations in the cerebrospinal fluid of alzheimer’s disease patients. Front. Neurosci. 2019, 13, 871. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1alpha,25-dihydroxyVitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef] [Green Version]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of Vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [Green Version]
- Bozic, M.; Guzman, C.; Benet, M.; Sanchez-Campos, S.; Garcia-Monzon, C.; Gari, E.; Gatius, S.; Valdivielso, J.M.; Jover, R. Hepatocyte Vitamin D receptor regulates lipid metabolism and mediates experimental diet-induced steatosis. J. Hepatol. 2016, 65, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Kuchenbecker, J.; Grosgen, S.; Burg, V.K.; Hundsdorfer, B.; Rothhaar, T.L.; Friess, P.; de Wilde, M.C.; Broersen, L.M.; Penke, B.; et al. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J. Biol. Chem. 2011, 286, 14028–14039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, M.O.; Mett, J.; Stahlmann, C.P.; Haupenthal, V.J.; Blumel, T.; Stotzel, H.; Grimm, H.S.; Hartmann, T. Eicosapentaenoic acid and docosahexaenoic acid increase the degradation of amyloid-beta by affecting insulin-degrading enzyme. Biochem. Cell Biol. 2016, 94, 534–542. [Google Scholar] [CrossRef]
- Grimm, M.O.; Haupenthal, V.J.; Mett, J.; Stahlmann, C.P.; Blumel, T.; Mylonas, N.T.; Endres, K.; Grimm, H.S.; Hartmann, T. Oxidized docosahexaenoic acid species and lipid peroxidation products increase amyloidogenic amyloid precursor protein processing. Neurodegener. Dis. 2016, 16, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Bunner, A.E.; Agarwal, U. Saturated and trans fats and dementia: A systematic review. Neurobiol. Aging 2014, 35 (Suppl. S2), S65–S73. [Google Scholar] [CrossRef] [Green Version]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Dorninger, F.; Moser, A.B.; Kou, J.; Wiesinger, C.; Forss-Petter, S.; Gleiss, A.; Hinterberger, M.; Jungwirth, S.; Fischer, P.; Berger, J. Alterations in the plasma levels of specific choline phospholipids in alzheimer’s disease mimic accelerated aging. J. Alzheimer’s Dis. 2018, 62, 841–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Mejia, R.O.; Mucke, L. Phospholipase a2 and arachidonic acid in alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Mejia, R.O.; Newman, J.W.; Toh, S.; Yu, G.Q.; Zhou, Y.; Halabisky, B.; Cisse, M.; Scearce-Levie, K.; Cheng, I.H.; Gan, L.; et al. Phospholipase a2 reduction ameliorates cognitive deficits in a mouse model of alzheimer’s disease. Nat. Neurosci. 2008, 11, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, V.; Goodenowe, D.B. Plasmalogen deficiency and neuropathology in alzheimer’s disease: Causation or coincidence? Alzheimer’s Dement. 2019, 5, 524–532. [Google Scholar] [CrossRef]
- Jenkins, C.M.; Yang, K.; Liu, G.; Moon, S.H.; Dilthey, B.G.; Gross, R.W. Cytochrome c is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage. J. Biol. Chem. 2018, 293, 8693–8709. [Google Scholar] [CrossRef] [Green Version]
- Rothhaar, T.L.; Grosgen, S.; Haupenthal, V.J.; Burg, V.K.; Hundsdorfer, B.; Mett, J.; Riemenschneider, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O. Plasmalogens inhibit app processing by directly affecting gamma-secretase activity in alzheimer’s disease. Sci. World J. 2012, 2012, 141240. [Google Scholar] [CrossRef] [Green Version]

| Lipid Species | Vitamin D Deficiency | Selection of Proposed AD-Related Mechanisms |
|---|---|---|
| PUFA | ↓ | PUFAs are reported to be decreased in AD due to increased ROS levels. Moreover, in particular DHA is known to affect Aβ production via multiple pleiotropic mechanisms, including an increase of nonamyloidogenic processing by increase of ADAM17 protein levels accompanied by an increase in ADAM17 expression, and a decrease in ADAM17 protein degradation. Furthermore, amyloidogenic APP processing is decreased in presence of PUFAs, resulting in reduced Aβ production. Underlying mechanisms include a decreased BACE1 activity; a changed distribution of BACE1 on the cell surface/intracellular BACE1 pool is discussed. With respect to γ-secretase, a direct effect of DHA on enzyme activity is reported, accompanied by a shift from γ-secretase from raft to nonraft [80]. Further potential mechanisms include effects on Aβ degradation. In particular, EPA has been shown to increase IDE mediated Aβ degradation [81]. Importantly, oxidized PUFA species seem to have controversial effects [82]. |
| SFA | ↑ | Saturated fatty acids are reported to be linked with dementia in several studies, reviewed, e.g., in [83]. Potential molecular mechanisms might include an effect of SFA on α-secretase and membrane fluidity (SFAs show a decrease on α-secretase activity and membrane fluidity compared to MUFAs and PUFAs) [64]. In return, SFAs seem to increase β-amyloid secretion and are associated with reduced β-amyloid elimination. Moreover, SFAs are discussed to be associated with an increase in diabetes and insulin resistance, which play a crucial role in AD [84]. |
| Lyso-PC | ↑ | Lyso-PCs are reported to be increased during aging and in particular in AD [43,85]. Importantly, a tight link between phospholipase A2, resulting in lyso-PC generation, and Aβ has been reported [86]. In line, PLA2 reduction was shown to ameliorate cognitive deficits in AD mouse models [87]. Additionally, lyso-PC increases neurotoxicity of Aβ1-42, and a potential impact of Aβ oligomerization induced by lyso PCs is discussed [74]. |
| Plasmalogens | ↑ | Plasmalogens are known to be decreased in AD brains [88]. Plasmalogens are known to be vulnerable to ROS species, being increased in AD [89]. In return, plasmalogens decrease Aβ generation [90]. Importantly, vitamin D3 deficiency results in an increase in plasmalogens both in murine brain and in cell culture. Further experiments are needed to proof whether vitamin D3 supplementation in human results in decreased plasmalogens levels, which would be unfavorable with respect to AD. Therefore, an additional plasmalogens—in addition to vitamin D3 supplementation—might be useful and should be further investigated with respect to AD. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauer, A.A.; Griebsch, L.V.; Pilz, S.M.; Janitschke, D.; Theiss, E.L.; Reichrath, J.; Herr, C.; Beisswenger, C.; Bals, R.; Valencak, T.G.; et al. Impact of Vitamin D3 Deficiency on Phosphatidylcholine-/Ethanolamine, Plasmalogen-, Lyso-Phosphatidylcholine-/Ethanolamine, Carnitine- and Triacyl Glyceride-Homeostasis in Neuroblastoma Cells and Murine Brain. Biomolecules 2021, 11, 1699. https://doi.org/10.3390/biom11111699
Lauer AA, Griebsch LV, Pilz SM, Janitschke D, Theiss EL, Reichrath J, Herr C, Beisswenger C, Bals R, Valencak TG, et al. Impact of Vitamin D3 Deficiency on Phosphatidylcholine-/Ethanolamine, Plasmalogen-, Lyso-Phosphatidylcholine-/Ethanolamine, Carnitine- and Triacyl Glyceride-Homeostasis in Neuroblastoma Cells and Murine Brain. Biomolecules. 2021; 11(11):1699. https://doi.org/10.3390/biom11111699
Chicago/Turabian StyleLauer, Anna Andrea, Lea Victoria Griebsch, Sabrina Melanie Pilz, Daniel Janitschke, Elena Leoni Theiss, Jörg Reichrath, Christian Herr, Christoph Beisswenger, Robert Bals, Teresa Giovanna Valencak, and et al. 2021. "Impact of Vitamin D3 Deficiency on Phosphatidylcholine-/Ethanolamine, Plasmalogen-, Lyso-Phosphatidylcholine-/Ethanolamine, Carnitine- and Triacyl Glyceride-Homeostasis in Neuroblastoma Cells and Murine Brain" Biomolecules 11, no. 11: 1699. https://doi.org/10.3390/biom11111699
APA StyleLauer, A. A., Griebsch, L. V., Pilz, S. M., Janitschke, D., Theiss, E. L., Reichrath, J., Herr, C., Beisswenger, C., Bals, R., Valencak, T. G., Portius, D., Grimm, H. S., Hartmann, T., & Grimm, M. O. W. (2021). Impact of Vitamin D3 Deficiency on Phosphatidylcholine-/Ethanolamine, Plasmalogen-, Lyso-Phosphatidylcholine-/Ethanolamine, Carnitine- and Triacyl Glyceride-Homeostasis in Neuroblastoma Cells and Murine Brain. Biomolecules, 11(11), 1699. https://doi.org/10.3390/biom11111699









