Overweight as a Favorable Clinical Biomarker for Checkpoint Inhibitor Therapy Response in Recurrent Gynecologic Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Descriptive Characteristics
3.2. Predictive Value of a Pretherapeutic BMI for CPI Therapy
3.3. Prognostic Value of a Pretherapeutic BMI on Patient Survival during CPI Therapy
3.4. Subgroup Analysis Excluding Vaginal Cancer Patients
3.5. Immune-Related Adverse Events (irAEs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roche Provides Update on Phase III Study of Tecentriq in Women with Advanced Stage Ovarian Cancer. News Release. Roche. A.A., 13 July 2021. bit.ly/2CsVGpV. Available online: https://www.roche.com/media/releases/med-cor-2020-07-13.htm (accessed on 19 October 2021).
- Ledermann, J.A.; Colombo, N.; Oza, A.M.; Fujiwara, K.; Birrer, M.J.; Randall, L.M.; Poddubskaya, E.V.; Scambia, G.; Shparyk, Y.V.; Lim, M.C.; et al. Avelumab in combination with and/or following chemotherapy vs chemotherapy alone in patients with previously untreated epithelial ovarian cancer: Results from the phase 3 javelin ovarian 100 trial. Gynecol. Oncol. 2020, 159, 13–14. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- O’Malley, D.; Marabelle, A.; De Jesus-Acosta, A.; Piha-Paul, S.A.; Arkhipov, A.; Longo, F.; Motola-Kuba, D.; Shapira-Frommer, R.; Geva, R.; Rimel, B.J.; et al. 1044P–Pembrolizumab in patients with MSI-H advanced endometrial cancer from the KEYNOTE-158 study. Ann. Oncol. 2019, 30, v425–v426. [Google Scholar] [CrossRef]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.-P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical activity and safety of the anti–programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair–deficient endometrial cancer: A nonrandomized phase 1 clinical trial. JAMA Oncol. 2020, 6, 1766–1772. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet. Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Bravaccini, S. What impacts the cost-effectiveness of PD-L1 testing in non-small cell lung cancer? Lung Cancer 2019, 132, 152–153. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Kichenadasse, G.; Miners, J.O.; Mangoni, A.A.; Rowland, A.; Hopkins, A.M.; Sorich, M.J. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020, 6, 512–518. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- Oberndorfer, F.; Moling, S.; Hagelkruys, L.A.; Grimm, C.; Polterauer, S.; Sturdza, A.; Aust, S.; Reinthaller, A.; Müllauer, L.; Schwameis, R. Risk reclassification of patients with endometrial cancer based on tumor molecular profiling: First real world data. J. Pers. Med. 2021, 11, 48. [Google Scholar] [CrossRef]
- Persigehl, T.; Lennartz, S.; Schwartz, L.H. iRECIST: How to do it. Cancer Imaging 2020, 20, 2. [Google Scholar] [CrossRef]
- Bekos, C.; Pils, D.; Dekan, S.; Hofstetter, G.; Horak, P.; Reinthaller, A.; Polterauer, S.; Schwameis, R.; Aust, S. PD-1 and PD-L1 expression on TILs in peritoneal metastases compared to ovarian tumor tissues and its associations with clinical outcome. Sci. Rep. 2021, 11, 6400. [Google Scholar] [CrossRef]
- Kulangara, K.; Hanks, D.A.; Waldroup, S.; Peltz, L.; Shah, S.; Roach, C.; Juco, J.W.; Emancipator, K.; Stanforth, D. Development of the combined positive score (CPS) for the evaluation of PD-L1 in solid tumors with the immunohistochemistry assay PD-L1 IHC 22C3 pharmDx. J. Clin. Oncol. 2017, 35, e14589. [Google Scholar] [CrossRef]
- Nattenmüller, J.; Wochner, R.; Muley, T.; Steins, M.; Hummler, S.; Teucher, B.; Wiskemann, J.; Kauczor, H.U.; Wielpütz, M.O.; Heussel, C.P. Prognostic impact of CT-quantified muscle and fat distribution before and after first-line-chemotherapy in lung cancer patients. PLoS ONE 2017, 12, e0169136. [Google Scholar] [CrossRef]
- Suidan, R.S.; Leitao, M.M., Jr.; Zivanovic, O.; Gardner, G.J.; Long Roche, K.C.; Sonoda, Y.; Levine, D.A.; Jewell, E.L.; Brown, C.L.; Abu-Rustum, N.R.; et al. Predictive value of the Age-Adjusted Charlson Comorbidity Index on perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian cancer. Gynecol. Oncol. 2015, 138, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Browne, I.; Fennelly, D.W.; Crown, J.; Murray, H. The efficacy and safety of pembrolizumab in advanced cervical cancer—A real world treatment study in an irish healthcare setting. J. Clin. Oncol. 2020, 38, e18007. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef]
- How, J.A.; Jazaeri, A.A.; Soliman, P.T.; Fleming, N.D.; Gong, J.; Piha-Paul, S.A.; Janku, F.; Stephen, B.; Naing, A. Pembrolizumab in vaginal and vulvar squamous cell carcinoma: A case series from a phase II basket trial. Sci. Rep. 2021, 11, 3667. [Google Scholar] [CrossRef]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The obesity paradox in cancer: A review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Wu, B.; Sun, X.; Gupta, H.B.; Yuan, B.; Li, J.; Ge, F.; Chiang, H.-C.; Zhang, X.; Zhang, C.; Zhang, D.; et al. Adipose PD-L1 modulates PD-1/PD-L1 checkpoint blockade immunotherapy efficacy in breast cancer. Oncoimmunology 2018, 7, e1500107. [Google Scholar] [CrossRef] [Green Version]
- Woodall, M.J.; Neumann, S.; Campbell, K.; Pattison, S.T.; Young, S.L. The effects of obesity on anti-cancer immunity and cancer immunotherapy. Cancers 2020, 12, 1230. [Google Scholar] [CrossRef]
- An, Y.; Wu, Z.; Wang, N.; Yang, Z.; Li, Y.; Xu, B.; Sun, M. Association between body mass index and survival outcomes for cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. J. Transl. Med. 2020, 18, 235. [Google Scholar] [CrossRef]
- Young, A.C.; Quach, H.T.; Song, H.; Davis, E.J.; Moslehi, J.J.; Ye, F.; Williams, G.R.; Johnson, D.B. Impact of body composition on outcomes from anti-PD1+/− anti-CTLA-4 treatment in melanoma. J. Immunother. Cancer 2020, 8, e000821. [Google Scholar] [CrossRef]
- Khaddour, K.; Gomez-Perez, S.L.; Jain, N.; Patel, J.D.; Boumber, Y. Obesity, sarcopenia, and outcomes in non-small cell lung cancer patients treated with immune checkpoint inhibitors and tyrosine kinase inhibitors. Front. Oncol. 2020, 10, 576314. [Google Scholar] [CrossRef]

| Parameter | All Patients | BMI < 25 | BMI ≥ 25 | p-Value |
|---|---|---|---|---|
| number of patients | 36 | 20 | 16 | 0.679 * |
| age at CPI induction (years) | 56.5 (45.8–65.8) | 54.5 (40.8–62.8) | 58.5 (52.0–68.3) | 0.679 * |
| CPI courses administered | 8 (5–15) | 5 (5–10) | 10 (7–23) | 0.042 * |
| primary | 0.578 † | |||
| endometrium | 9 (25.0%) | 4 (20.0%) | 5 (31.3%) | |
| cervix | 21 (58.3%) | 12 (60.0%) | 9 (56.3%) | |
| vulva | 4 (11.1%) | 2 (10.0%) | 2 (12.5%) | |
| vagina | 2 (5.6%) | 2 (10.0%) | 0 (0%) | |
| body mass index (BMI) | 24.7 (20.5–27.6) | 21.4 (18.8–24.3) | 27.7 (26.3–32.9) | <0.001 * |
| combined positive score (CPS) | 30.0 (5.0–72.5) | 35.0 (7.5–84.0) | 30.0 (3.5–72.5) | 0.639 * |
| charlson comorbidity index | 7 (6–8) | 7 (6–8) | 7 (7–8) | 0.406 * |
| neutrophile-to-platelet ratio | 5.1 (3.6–10.0) | 4.9 (3.8–9.4) | 9.1 (2.8–11.1) | 0.868 * |
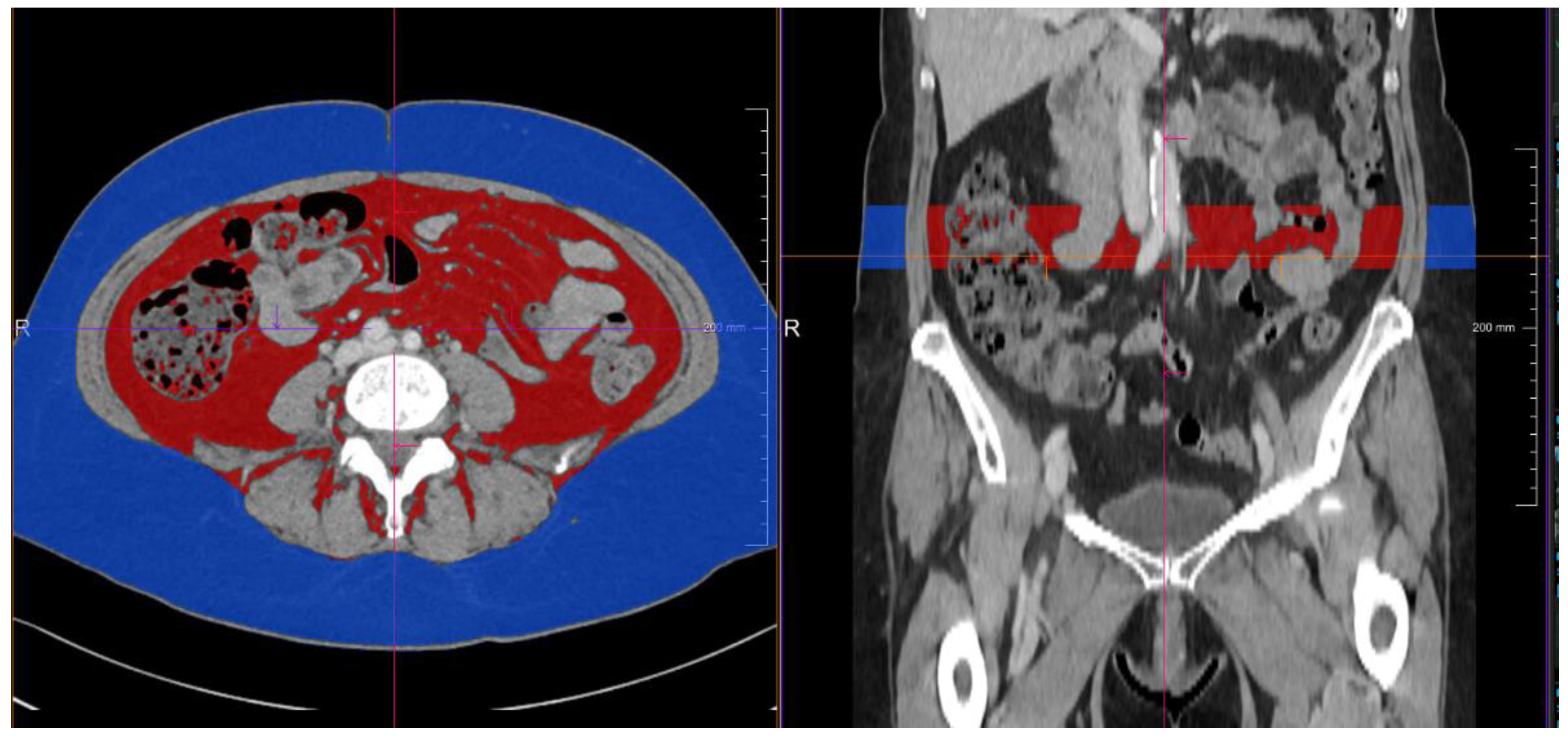
| CT-derived subcutaneous fat volume (mL) | 711.8 (334.8–1202.3) | 476.7 (223.0–721.7) | 1242.9 (904.6–1534.1) | <0.001 * |
| CT-based visceral fat volume (mL) | 327.1 (160.1–557.2) | 226.3 (99.5–388.7) | 532.4 (285.2–755.4) | <0.001 * |
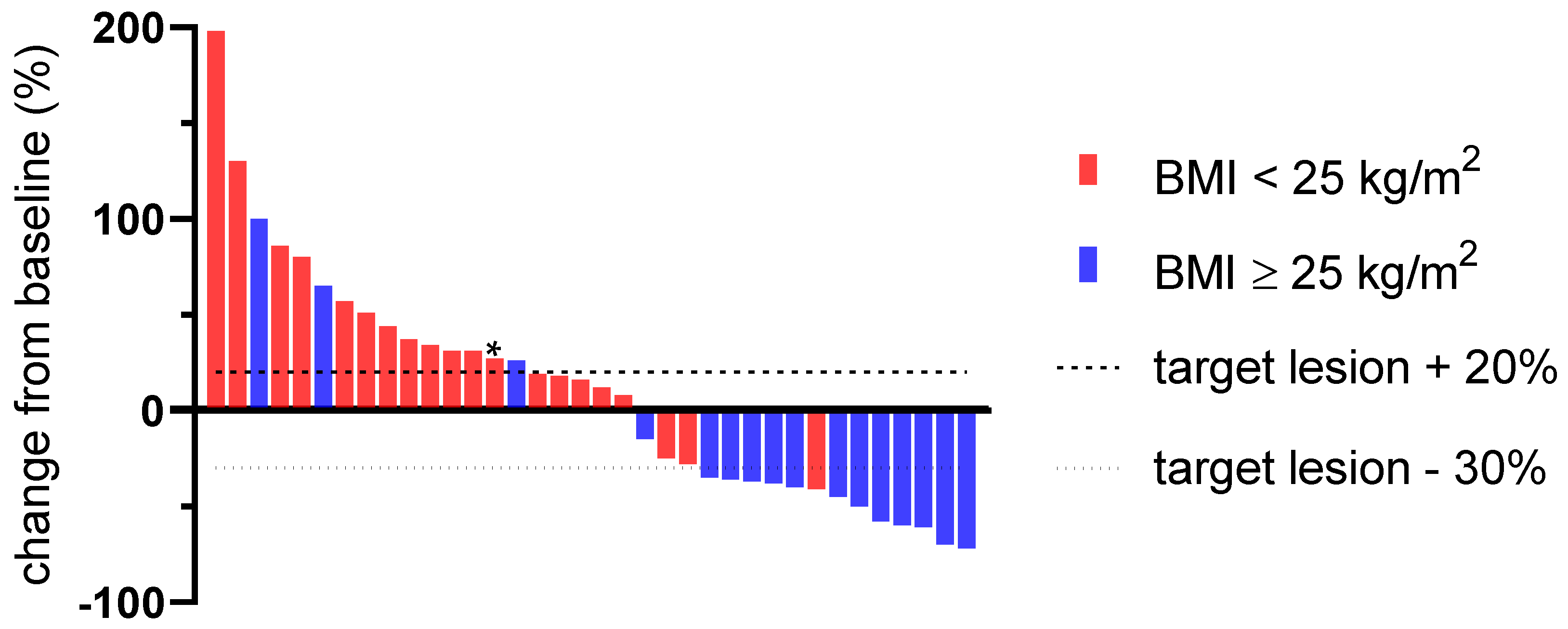
| overall response rate (ORR) | 36.1% (13/36) | 5.0% (1/20) | 75.0% (12/16) | <0.001 * |
| disease control rate (DCR) | 58.3% (21/36) | 40.0% (8/20) | 81.3% (13/16) | 0.023 * |
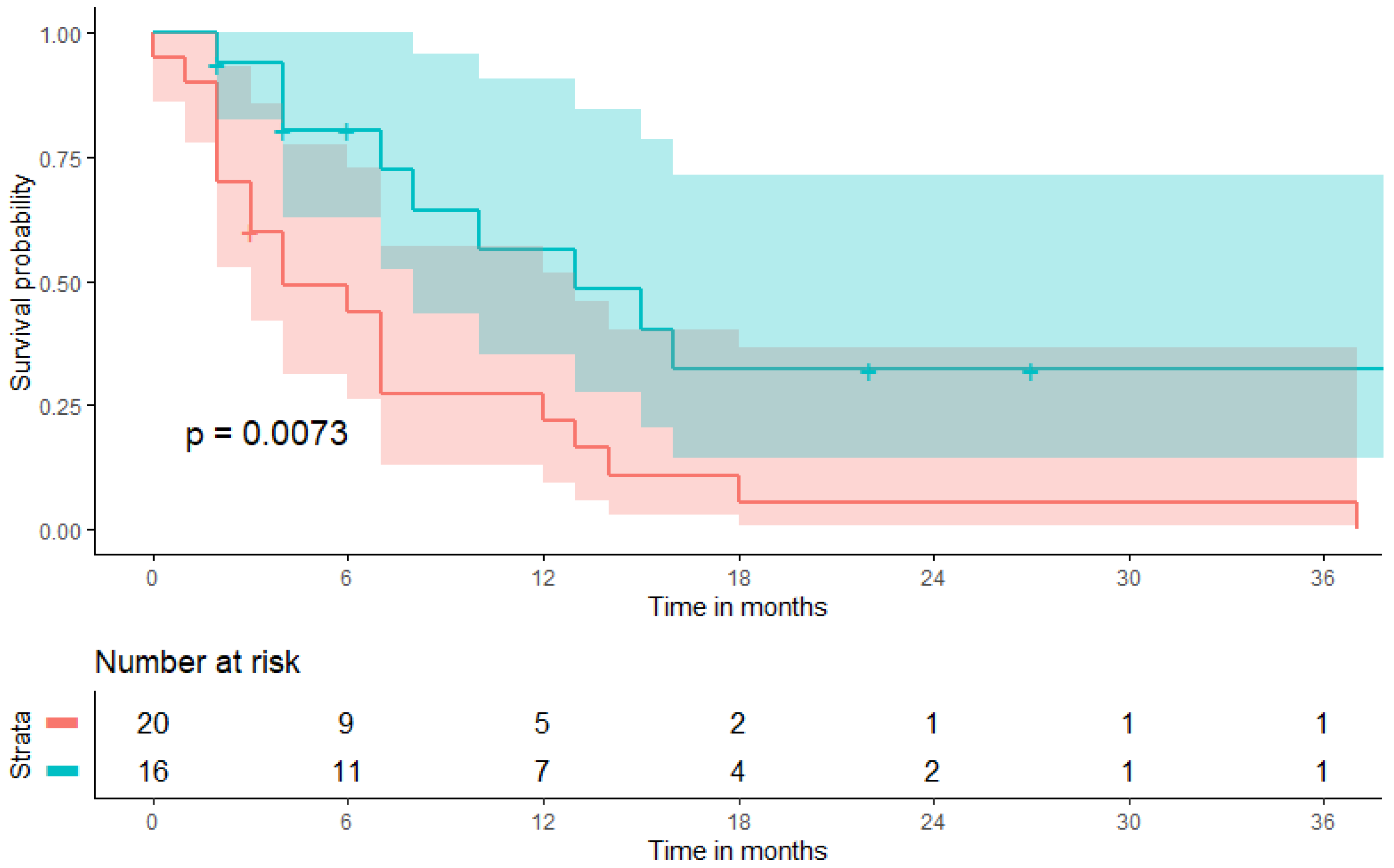
| progression-free survival (PFS, months) | 6.5 (3.0–13.8) | 4.0 (2.0–10.8) | 9.0 (4.0–20.5) | 0.102 * |
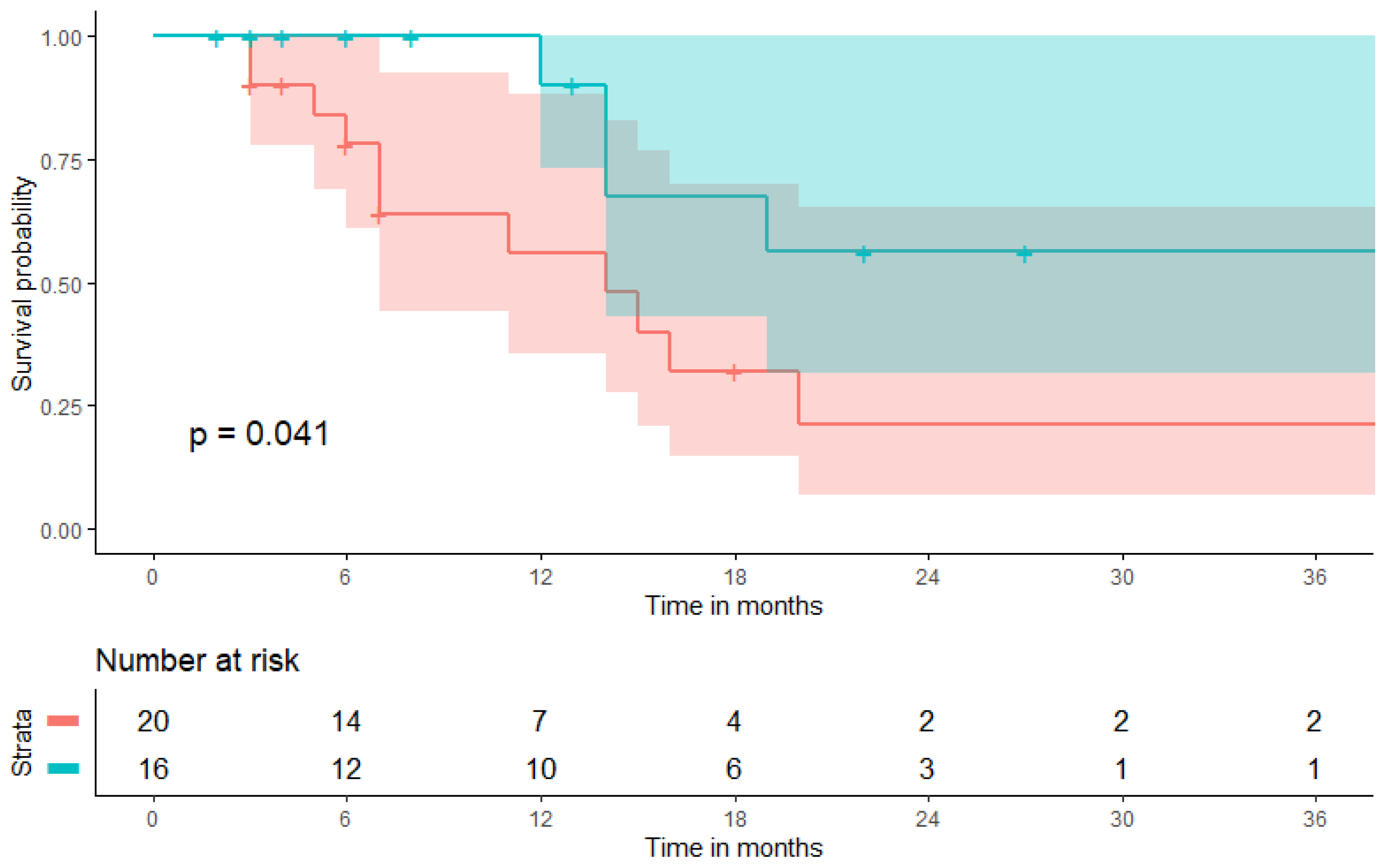
| overall survival (OS, months) | 9.5 (4.3–18.8) | 7.0 (4.3–15.8) | 13.5 (4.5–22.0) | 0.499 * |
| a. Parameters | Overall Response after CPI Therapy | |||
| Univariate Analysis | Multivariable Analysis | |||
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | |
| combined positive score | 0.608 | 0.99 (0.97–1.02) | - | - |
| body mass index (5 kg/m2 increment) | 0.002 | 10.93 (2.39–49.82) | 0.020 | 64.09 (1.90–2160.48) |
| neutrophile-to-lymphocyte ratio | 0.572 | 0.95 (0.81–1.13) | 0.681 | 0.94 (0.68–1.28) |
| age-adjusted charlson comorbidity index | 0.373 | 1.19 (0.81–1.74) | 0.418 | 0.71 (0.31–1.62) |
| subcutaneous fat volume (100 mL increment) | 0.023 | 1.20 (1.03–1.41) | 0.186 | 0.712 (0.44–1.17) |
| visceral fat volume (100 mL increment) | 0.108 | 1.26 (0.95–1.66) | - | - |
| b. Parameters | Disease Control after CPI Therapy | |||
| Univariate Analysis | Multivariable Analysis | |||
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | |
| combined positive score | 0.163 | 0.98 (0.96–1.01) | ||
| body mass index (5 kg/m2 increment) | 0.048 | 2.19 (0.99–4.83) | 0.026 | 10.07 (1.33–76.51) |
| neutrophile-to-lymphocyte ratio | 0.144 | 0.89 (0.76–1.04) | 0.199 | 0.87 (0.51–1.40) |
| age-adjusted charlson comorbidity index | 0.968 | 0.972 (0.241–3.93) | 0.506 | 0.84 (0.51–1.40) |
| subcutaneous fat volume (100 mL increment) | 0.745 | 1.02 (0.89–1.17) | 0.063 | 0.720 (0.51–1.02) |
| visceral fat volume (100 mL increment) | 0.474 | 1.10 (0.84–1.44) | - | - |
| a. Parameters | PFS after CPI Therapy | |||
| Univariate Analysis | Multivariable Analysis | |||
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | |
| combined positive score | 0.746 | 1.00 (0.99–1.01) | - | - |
| body mass index (5 kg/m2 increment) | 0.038 | 1.54 (1.03–2.34) | 0.002 | 3.73 (1.63–8.50) |
| neutrophile-to-lymphocyte ratio | 0.767 | 1.01 (0.95–1.08) | 0.789 | 0.99 (0.93–1.06) |
| age-adjusted charlson comorbidity index | 0.675 | 1.04 (0.85–1.28) | 0.419 | 1.11 (0.87–1.41) |
| subcutaneous fat volume (100 mL increment) | 0.992 | 1.00 (0.92–1.08) | 0.007 | 1.23 (1.06–1.43) |
| visceral fat volume (100 mL increment) | 0.487 | 0.95 (0.82–1.10) | - | - |
| b. Parameters | OS after CPI Therapy | |||
| Univariate Analysis | Multivariable Analysis | |||
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | |
| combined positive score | 0.220 | 1.01 (0.991.–1.03) | - | - |
| body mass index (5 kg/m2 increment) | 0.028 | 1.87 (1.07–3.29) | 0.010 | 7.44 (1.62–34.16) |
| neutrophile-to-lymphocyte ratio | 0.397 | 1.04 (0.95–1.15) | 0.478 | 1.04 (0.94–1.14) |
| age-adjusted charlson comorbidity index | 0.959 | 0.99 (0.73–1.36) | 0.694 | 1.07 (0.75–1.53) |
| subcutaneous fat volume (100 mL increment) | 0.620 | 0.973 (0.873–1.08) | 0.035 | 1.36 (1.02–1.81) |
| visceral fat volume (100 mL increment) | 0.201 | 0.868 (0.70–1.08) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartl, T.; Onoprienko, A.; Hofstetter, G.; Müllauer, L.; Poetsch, N.; Fuereder, T.; Kofler, P.; Polterauer, S.; Grimm, C. Overweight as a Favorable Clinical Biomarker for Checkpoint Inhibitor Therapy Response in Recurrent Gynecologic Cancer Patients. Biomolecules 2021, 11, 1700. https://doi.org/10.3390/biom11111700
Bartl T, Onoprienko A, Hofstetter G, Müllauer L, Poetsch N, Fuereder T, Kofler P, Polterauer S, Grimm C. Overweight as a Favorable Clinical Biomarker for Checkpoint Inhibitor Therapy Response in Recurrent Gynecologic Cancer Patients. Biomolecules. 2021; 11(11):1700. https://doi.org/10.3390/biom11111700
Chicago/Turabian StyleBartl, Thomas, Arina Onoprienko, Gerda Hofstetter, Leonhard Müllauer, Nina Poetsch, Thorsten Fuereder, Paul Kofler, Stephan Polterauer, and Christoph Grimm. 2021. "Overweight as a Favorable Clinical Biomarker for Checkpoint Inhibitor Therapy Response in Recurrent Gynecologic Cancer Patients" Biomolecules 11, no. 11: 1700. https://doi.org/10.3390/biom11111700









