The Synthesis and Biological Evaluation of D-Ring-Modified Vitamin D Analogues
Abstract
:1. Introduction
2. 16-Ene-Vitamin D3 Analogues
3. 16-Modified Vitamin D3 Analogues
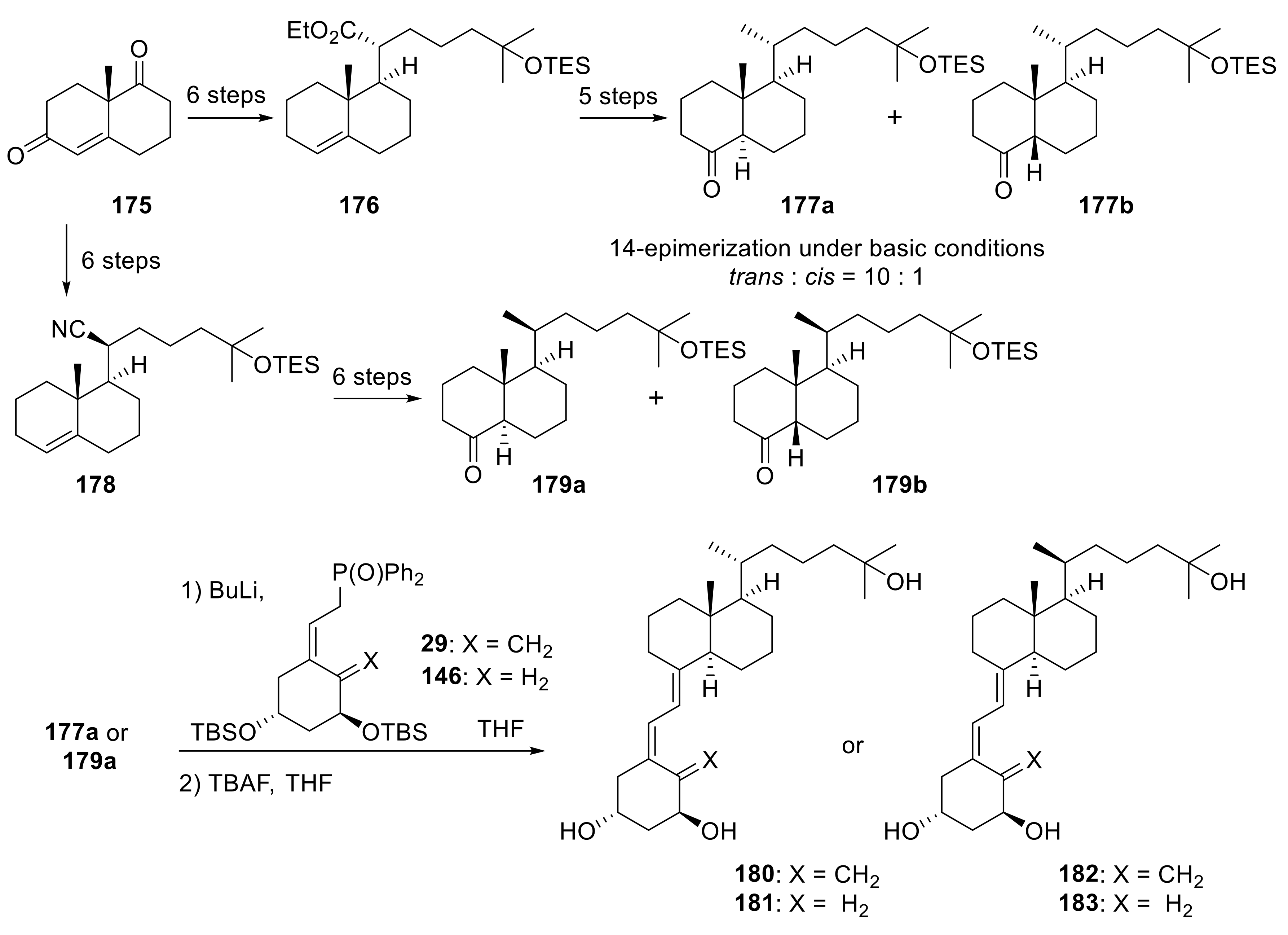
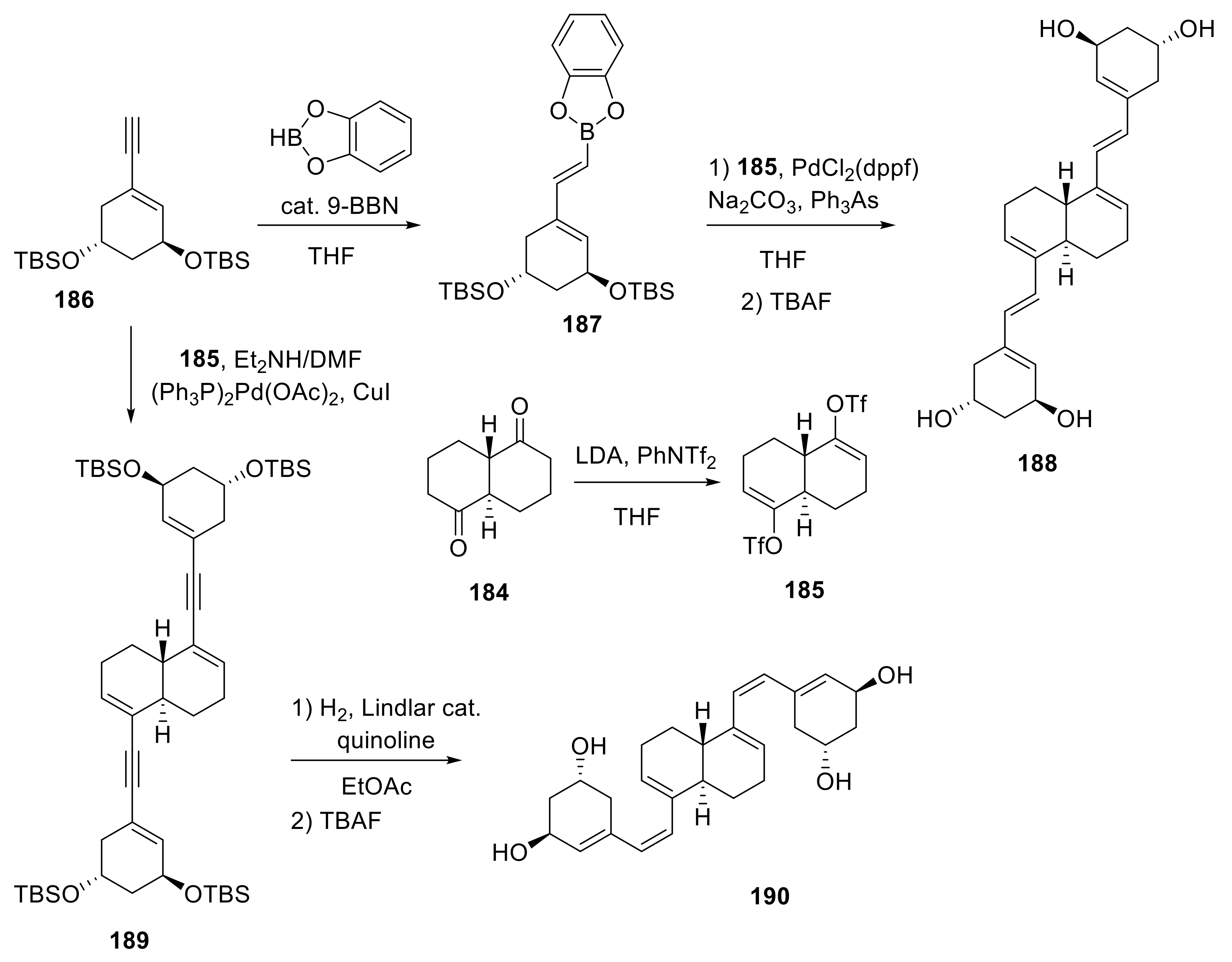
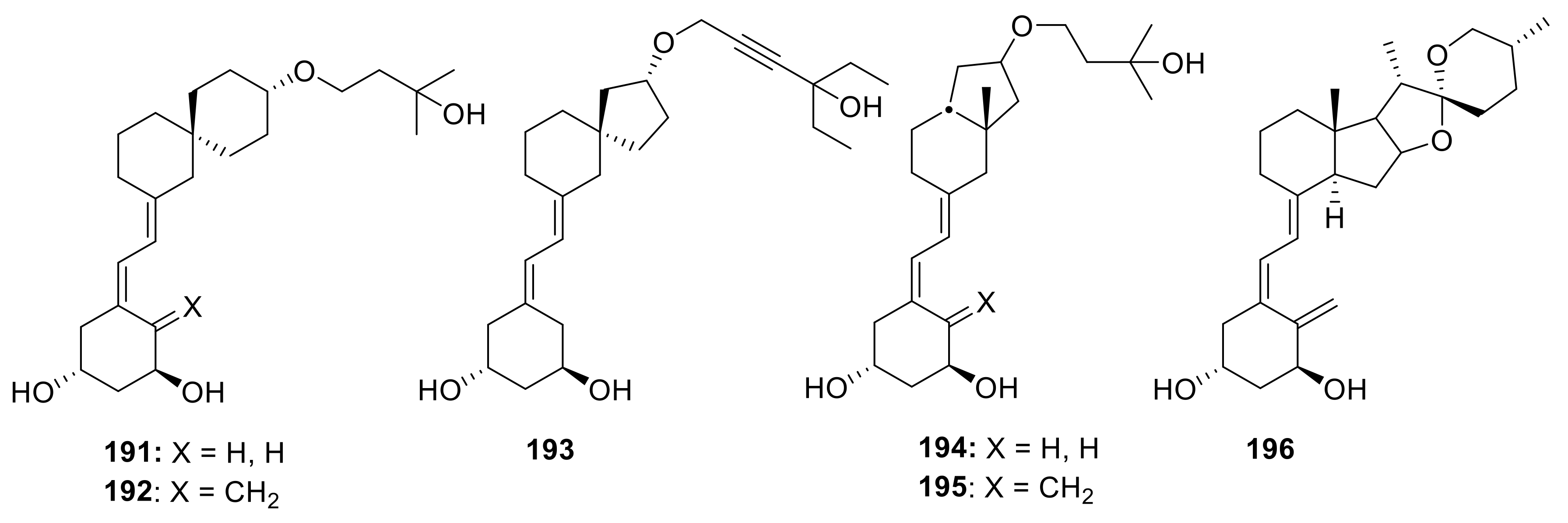
4. Decalin-Vitamin D analogues
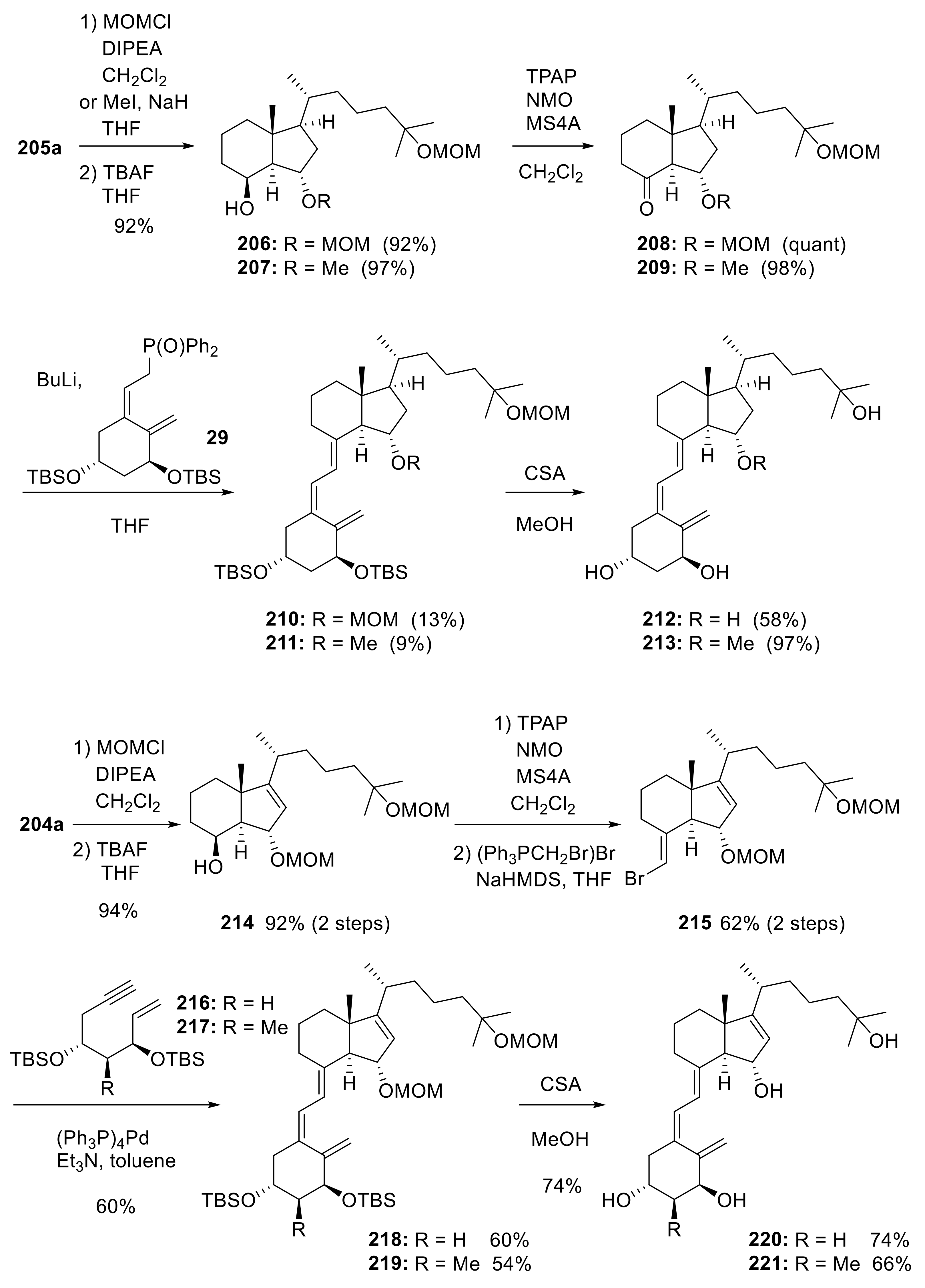
5. 15-Substituted Vitamin D3 Analogues
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Posner, G.H.; Kahraman, M. Overview: Rational design of 1α,25-dihydroxyvitamin D3 analogs (Deltanoids). In Vitamin D, 2nd ed.; Feldman, D., Pike, J.W., Glorieux, F.H., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2005; Volume 2, pp. 1405–1422. [Google Scholar]
- Kulesza, U.; Sigüeiro, R.; Mouriño, A.; Sicinski, R.R. Synthesis of 9-alkylated calcitriol and two 1α,25-dihydroxy-9-methylene-10,19-dihydrovitamin D3 analogues with a non-natural triene system by thermal sigmatropic rearrangements. J. Org. Chem. 2013, 78, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Rochel, N.; Wurtz, J.M.; Mitschler, A.; Klaholz, B.; Moras, D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell 2000, 5, 173–179. [Google Scholar] [CrossRef]
- Sakaki, T.; Sawada, N.; Komai, K.; Shiozawa, S.; Yamada, S.; Yamamoto, K.; Ohyama, Y.; Inouye, K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur. J. Biochem. 2000, 267, 6158–6165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaki, T.; Kagawa, N.; Yamamoto, K.; Inouye, K. Metabolism of vitamin D3 by cytochromes P450. Front. Biosci. 2005, 10, 119–134. [Google Scholar] [PubMed] [Green Version]
- Yasuda, K.; Nishikawa, M.; Okamoto, K.; Horibe, K.; Mano, H.; Yamaguchi, M.; Okon, R.; Nakagawa, K.; Tsugawa, N.; Okano, T.; et al. Elucidation of metabolic pathways of 25-hydroxyvitamin D3 mediated by CYP24A1 and CYP3A using Cyp24a1 knockout rats generated by CRISPR/Cas9 system. J. Biol. Chem. 2021, 296, 100668. [Google Scholar] [CrossRef] [PubMed]
- Ordentlich, P.; Heyman, R.A. Nonsteroidal Analogs. In Vitamin D, 2nd ed.; Feldman, D., Pike, J.W., Glorieux, F.H., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2005; Volume 2, pp. 1557–1567. [Google Scholar]
- Kulesza, U.; Plum, L.A.; DeLuca, H.F.; Mouriño, A.; Sicinski, R.R. Novel 9-alkyl- and 9-alkylidene-substituted 1α,25-dihydroxyvitamin D3 analogues: Synthesis and biological examinations. J. Med. Chem. 2015, 58, 6237–6247. [Google Scholar] [CrossRef]
- Gogoi, P.; Seoane, S.; Sigüeiro, R.; Guiberteau, T.; Maestro, M.A.; Pérez-Fernández, R.; Rochel, N.; Mouriño, A. Aromatic-based design of highly active and noncalcemic vitamin D receptor agonists. J. Med. Chem. 2018, 61, 4928–4937. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, G.-D.; Van Haver, D.; Vandewalle, M.; De Clercq, P.J.; Verstuyf, A.; Bouillon, R. Synthesis, biological activity, and conformational analysis of four seco-D-15,19-bisnor-1α,25-dihydroxyvitamin D analogues, diastereomeric at C17 and C20. J. Med. Chem. 1999, 42, 3539–3556. [Google Scholar] [CrossRef]
- Okabe, M.; Sun, R.-C.; Scalone, M.; Jibilian, C.H.; Hutchings, S.D. Synthesis of 25-hydroxycholecalcifer-16-en-23-ynol: A potential antipsoriatic agent. J. Org. Chem. 1995, 60, 767–771. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Norman, A.W.; Chen, D.-L.; Sun, G.-W.; Uskokovic, M.; Koeffler, H.P. 1,25-Dihydroxy-16-ene-23-yne-vitamin D3 prolongs survival time of leukemic mice. Proc. Natl. Acad. Sci. USA 1990, 87, 3929–3932. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.A.; Lee, Y.Y.; Pakkala, S.; de Vos, S.; Elstner, E.; Norman, A.W.; Green, J.; Uskokovic, M.; Koeffler, H.P. 1,25(OH)2-16-ene-vitamin D3 is a potent antileukemic agent with low potential to cause hypercalcemia. Leuk. Res. 1994, 18, 453–463. [Google Scholar] [CrossRef]
- Rao, L.G.; Sutherland, M.K.; Reddy, G.S.; Siu-Caldera, M.-L.; Uskokovic, M.R.; Murray, T.M. Effects of 1α,25-dihydroxy-16ene, 23yne-vitamin D3 on osteoblastic function in human osteosarcoma SaOS-2 cells: Differentiation-stage dependence and modulation by 17-β estradiol. Bone 1996, 19, 621–627. [Google Scholar] [CrossRef]
- Chen, T.C.; Persons, K.; Uskokovic, M.R.; Horst, R.L.; Holick, M.F. An evaluation of 1,25-dihydroxyvitamin D3 analogues on the proliferation and differentiation of cultured human keratinocytes, calcium metabolism and the differentiation of human HL-60 cells. J. Nutr. Biochem. 1993, 4, 49–57. [Google Scholar] [CrossRef]
- Posner, G.H.; Lee, J.K.; White, M.C.; Hutchings, R.H.; Dai, H.; Kachinski, J.L.; Dolan, P.; Kensler, T.W. Antiproliferative hybrid analogs of the hormone 1α,25-dihydroxyvitamin D3: Design, synthesis, and preliminary biological evaluation. J. Org. Chem. 1997, 62, 3299–3314. [Google Scholar] [CrossRef] [PubMed]
- Honzawa, S.; Yamamoto, Y.; Yamashita, A.; Sugiura, T.; Kurihara, M.; Arai, M.A.; Kato, S.; Kittaka, A. The 2α-(3-hydroxypropyl) group as an active motif in vitamin D3 analogues as agonists of the mutant vitamin D receptor (Arg274Leu). Bioorg. Med. Chem. 2008, 16, 3002–3024. [Google Scholar] [CrossRef] [PubMed]
- Siu-Caldera, M.-L.; Clark, J.W.; Santos-Moore, A.; Peleg, S.; Liu, Y.Y.; Uskoković, M.R.; Sharma, S.; Reddy, G.S. 1α,25-dihydroxy-24-oxo-16-ene vitamin D3, a metabolite of a synthetic vitamin D3 analog, 1α,25-dihydroxy-16-ene vitamin D3, is equipotent to its parent in modulating growth and differentiation of human leukemic cells. J. Steroid Biochem. Mol. Biol. 1996, 59, 405–412. [Google Scholar] [CrossRef]
- Posner, G.H.; Lee, J.K.; Wang, Q.; Peleg, S.; Burke, M.; Brem, H.; Dolan, P.; Kensler, T.W. Noncalcemic, antiproliferative, transcriptionally active, 24-fluorinated hybrid analogues of the hormone 1α,25-dihydroxyvitamin D3. Synthesis and preliminary biological evaluation. J. Med. Chem. 1998, 41, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Posner, G.H.; Wang, Q.; Han, G.; Lee, J.K.; Crawford, K.; Zand, S.; Brem, H.; Peleg, S.; Dolan, P.; Kensler, T.W. Conceptually new sulfone analogues of the hormone 1α,25-dihydroxyvitamin D3: Synthesis and preliminary biological evaluation. J. Med. Chem. 1999, 42, 3425–3435. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, M.R.; Studzinski, G.P.; Gardner, J.P.; Reddy, S.G.; Campbell, M.J.; Koeffler, H.P. The 16-ene vitamin D analogs. Curr. Pharm. Des. 1997, 3, 99–123. [Google Scholar]
- Uskoković, M.R.; Studzinski, G.P.; Reddy, G.S. The 16-ene vitamin D analogs. In Vitamin D; Feldman, D., Glorieux, F.H., Pike, J.W., Eds.; Academic Press: New York, NY, USA, 1997; pp. 1045–1070. [Google Scholar]
- Rey, M.A.; Martínez-Pérez, J.A.; Fernández-Gacio, A.; Halkes, K.; Fall, Y.; Granja, J.; Mouriño, A. New synthetic strategies to vitamin D analogues modified at the side chain and D ring. Synthesis of 1α,25-dihydroxy-16-ene-vitamin D3 and C-20 analogues. J. Org. Chem. 1999, 64, 3196–3206. [Google Scholar]
- Fuchs, P.L.; Vedejs, E. Olefin inversion by the phosphorus betaine method. J. Am. Chem. Soc. 1971, 93, 4070–4072. [Google Scholar]
- Uskoković, M.R.; Manchand, P.; Marczak, S.; Maehr, H.; Jankowski, P.; Adorini, L.; Reddy, G.S. C-20 Cyclopropyl vitamin D3 analogs. Curr. Top. Med. Chem. 2006, 6, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Laverny, G.; Penna, G.; Uskoković, M.; Marczak, S.; Maehr, H.; Jankowski, P.; Ceailles, C.; Vouros, P.; Smith, B.; Robinson, M.; et al. Synthesis and anti-inflammatory properties of 1α,25-dihydroxy-16-ene-20-cyclopropyl-24-oxo-vitamin D3, a hypocalcemic, stable metabolite of 1α,25-dihydroxy-16-ene-20-cyclopropyl-vitamin D3. J. Med. Chem. 2009, 52, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Blæhr, L.K.A.; Björkling, F.; Calverley, M.J.; Binderup, E.; Begtrup, M. Synthesis of novel hapten derivatives of 1α,25-dihydroxyvitamin D3 and its 20-epi analogue. J. Org. Chem. 2003, 68, 1367–1375. [Google Scholar] [PubMed]
- Hosoda, H.; Sakai, Y.; Yoshida, H.; Miyairi, S.; Ishii, K.; Nambara, T. The preparation of steroid N-hydroxysuccinimide esters and their reactivities with bovine serum albumin. Chem. Pharm. Bull. 1979, 27, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibe, K.; Yamada, T.; Okamoto, S. Synthesis and vitamin D receptor affinity of 16-oxa vitamin D3 analogues. Org. Biomol. Chem. 2019, 17, 10188–10200. [Google Scholar] [CrossRef]
- Chen, Y.-J.; De Clercq, P.; Vandewalle, M. Synthesis of new vitamin D3 analogues with a decalin-type CD-ring. Tetrahedron Lett. 1996, 37, 9361–9364. [Google Scholar] [CrossRef]
- Lutz, F.T.; Utecht, J. Improved preparation of enantiomerically pure (+)-(4aS,5S)-5-tert-butoxy-4a-methyl-4,4a,5,6,7,8-hexahydro-2(3H)-naphthalenone. Synthesis 1993, 957–958. [Google Scholar]
- Chen, Y.-J.; Gao, L.-J.; Murad, I.; Verstuyf, A.; Verlinden, L.; Verboven, C.; Bouillon, R.; Viterbo, D.; Milanesio, M.; Van Haver, D.; et al. Synthesis, biological activity, and conformational analysis of CD-ring modified trans-decalin 1α,25-dihydroxyvitamin D analogs. Org. Biomol. Chem. 2003, 1, 257–267. [Google Scholar] [CrossRef]
- Minne, G.; Verlinden, L.; Verstuyf, A.; De Clercq, P.J. Synthesis of 1α,25-dihydroxyvitamin D analogues featuring a S2-symmetric CD-ring core. Molecules 2009, 14, 894–903. [Google Scholar] [CrossRef] [Green Version]
- Minne, G.B.; De Clercq, P.J. Synthesis of 3,4,7,8-tetrahydronaphthalene-1,5(2H,6H)-dione. Molecules 2007, 12, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schepens, W.; Van Haver, D.; Vandewalle, M.; De Clercq, P.J.; Bouillon, R.; Verstuyf, A. Synthesis and biological activity of 22-oxa CD-ring modified analogues of 1α,25-dihydroxyvitamin D3: Spiro[5.5]undecane CF-ring analogues. Bioorg. Med. Chem. Lett. 2004, 14, 3889–3892. [Google Scholar] [CrossRef] [PubMed]
- De Buysser, F.; Verlinden, L.; Verstuyf, A.; De Clercq, P.J. Synthesis of 22-oxaspiro[4.5]decane CD-ring modified analogs of 1α,25-dihydroxyvitamin D3. Tetrahedron Lett. 2009, 50, 4174–4177. [Google Scholar] [CrossRef]
- Demin, S.; Van Haver, D.; Vandewalle, M.; De Clercq, P.J.; Bouillon, R.; Verstuyf, A. Synthesis and biological activity of 22-oxa CD-ring modified analogues of 1α,25-dihydroxyvitamin D3: Cis-perhydrindane CE-ring analogues. Bioorg. Med. Chem. Lett. 2004, 14, 3885–3888. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.-J.; Koyanagi, J.; Komada, F.; Saito, S. Preparations of vitamin D analogs, spirostanols and furostanols from diosgenin and their cytotoxic activities. Eur. J. Med. Chem. 2005, 40, 662–673. [Google Scholar] [CrossRef]
- Verlinden, L.; Bouillon, R.; De Clercq, P.; Verstuyf, A. Analogs of Calcitriol. In Vitamin D, 4th ed.; Feldman, D., Pike, J.W., Bouillon, R., Giovannucci, E., Goltzman, D., Hewison, M., Eds.; Academic Press: London, UK, 2018; Volume 2, pp. 583–614. [Google Scholar]
- De Clercq, P.J.; De Buysser, F.; Minne, G.; Schepens, W.; Vrielynck, F.; van Haver, D.; Vandewalle, M.; Verstuyf, A.; Bouillon, R. The development of CD-ring modified analogs of 1α,25-dihydroxyvitamin D. J. Steroid Biochem. Mol. Biol. 2007, 103, 206–212. [Google Scholar] [CrossRef]
- Shindo, K.; Kumagai, G.; Takano, M.; Sawada, D.; Saito, N.; Saito, H.; Kakuda, S.; Takagi, K.; Ochiai, E.; Horie, K.; et al. New C15-Substituted Active Vitamin D3. Org. Lett. 2011, 13, 2852–2855. [Google Scholar] [CrossRef]
- Takahashi, T.; Ootake, A.; Tsuji, J.; Tachibana, K. Regio- and stereoselective introduction of 15β-hydroxy group and side chains to steroids by the palladium-catalyzed reaction of 1,3-diene monoepoxide. Tetrahedron 1985, 41, 5747–5754. [Google Scholar] [CrossRef]
- Marino, J.P.; Abe, H. New stereospecific approach to steroid side chains: Conversion of dehydroepiandrosterone to cholesterol, isocholesterol, and their 15β-hydroxy derivatives. J. Am. Chem. Soc. 1981, 103, 2907–2909. [Google Scholar] [CrossRef]
- Moon, S.; Stuhmiller, L.M.; Chadha, R.K.; McMorris, T.C. Synthesis of dehydro-oogoniol and oogoniol: The adrenosterone route. Tetrahedron 1990, 46, 2287–2306. [Google Scholar] [CrossRef]
- Konno, K.; Fujishima, T.; Maki, S.; Liu, Z.; Miura, D.; Chokki, M.; Ishizuka, S.; Yamaguchi, K.; Kan, Y.; Kurihara, M.; et al. Synthesis, biological evaluation, and conformational analysis of A-ring diastereomers of 2-methyl-1,25-dihydroxyvitamin D3 and their 20-epimers: Unique activity profiles depending on the stereochemistry of the A-ring and at C-20. J. Med. Chem. 2000, 43, 4247–4265. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Honzawa, S.; Kittaka, A. Recent results on A-ring modification of 1α,25-dihydroxyvitamin D3: Design and synthesis of VDR-agonists and antagonists with high biological activity. Curr. Top. Med. Chem. 2006, 6, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Hourai, S.; Fujishima, T.; Kittaka, A.; Suhara, Y.; Takayama, H.; Rochel, N.; Moras, D. Probing a water channel near the A-ring of receptor-bound 1α,25-dihydroxyvitamin D3 with selected 2α-substituted analogues. J. Med. Chem. 2006, 49, 5199–5205. [Google Scholar] [CrossRef] [PubMed]
- Honzawa, S.; Yamamoto, Y.; Hirasaka, K.; Takayama, H.; Kittaka, A. Synthesis of A-ring synthon of 2α-substituted vitamin D3 analogues utilizing Grignard reaction towards methyl 2,3-anhydro-4,6-O-benzylidene-α-D-manno-pyranoside. Heterocycles 2003, 61, 327–338. [Google Scholar] [CrossRef]
- Saito, N.; Matsunaga, T.; Saito, H.; Anzai, M.; Takenouchi, K.; Miura, D.; Namekawa, J.; Ishizuka, S.; Kittaka, A. Further synthetic and biological studies on vitamin D hormone antagonists based on C24-alkylation and C2α-functionalization of 25-dehydro-1α-hydroxyvitamin D3-26,23-lactones. J. Med. Chem. 2006, 49, 7063–7075. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, G.; Takano, M.; Shindo, K.; Sawada, D.; Saito, N.; Saito, H.; Kakuda, S.; Takagi, K.; Takimoto-Kamimura, M.; Takenouchi, K.; et al. C15-Functionalized 16-ene-1α,25-dihydroxyvitamin D3 is a new vitamin D analog with unique biological properties. Anticancer Res. 2012, 32, 311–318. [Google Scholar]
- Baggiolini, E.G.; Iacobelli, J.A.; Hennessy, B.M.; Uskoković, M.R. Stereoselective total synthesis of 1α,25-dihydroxycholecalciferol. J. Am. Chem. Soc. 1982, 104, 2945–2948. [Google Scholar] [CrossRef]




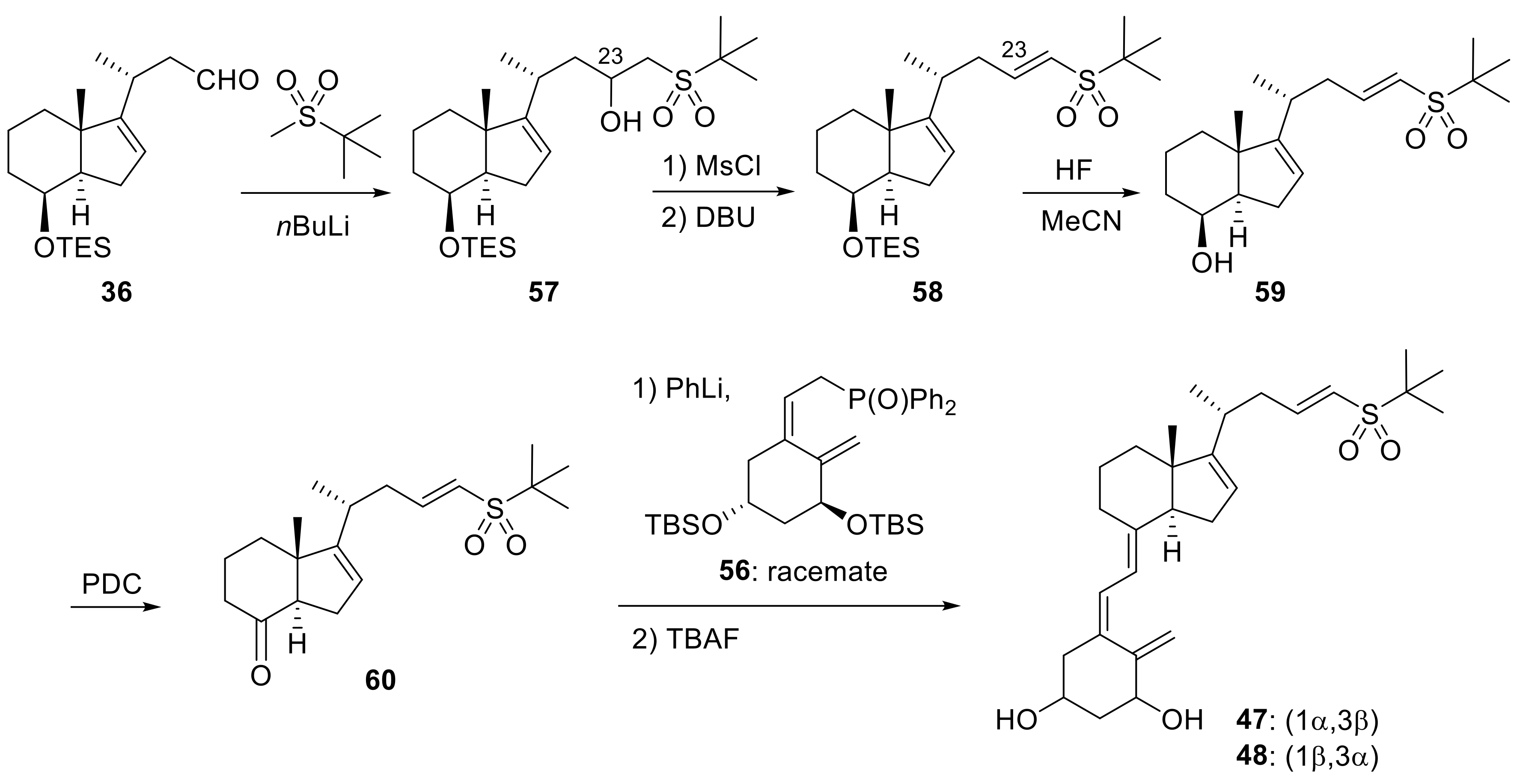
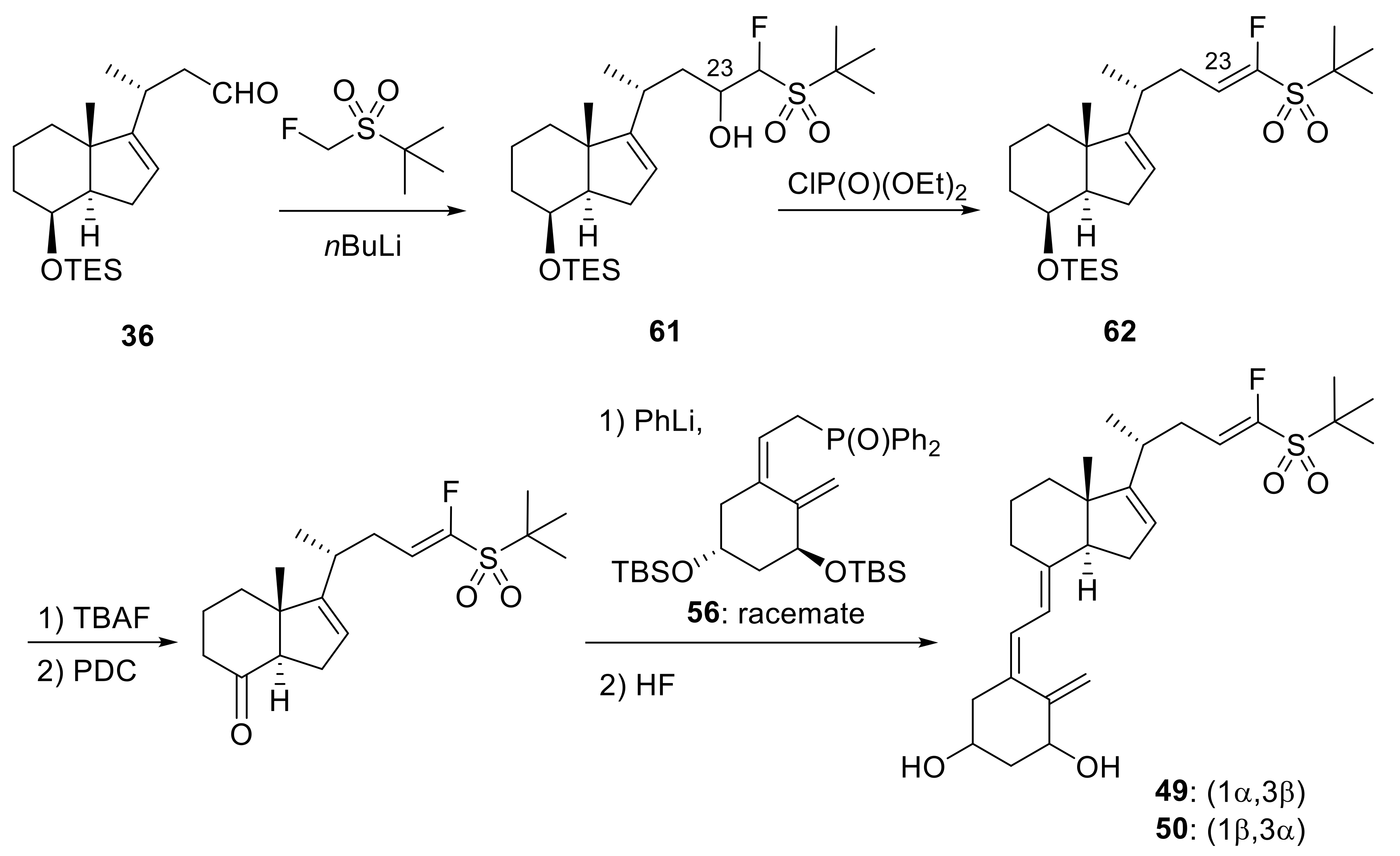
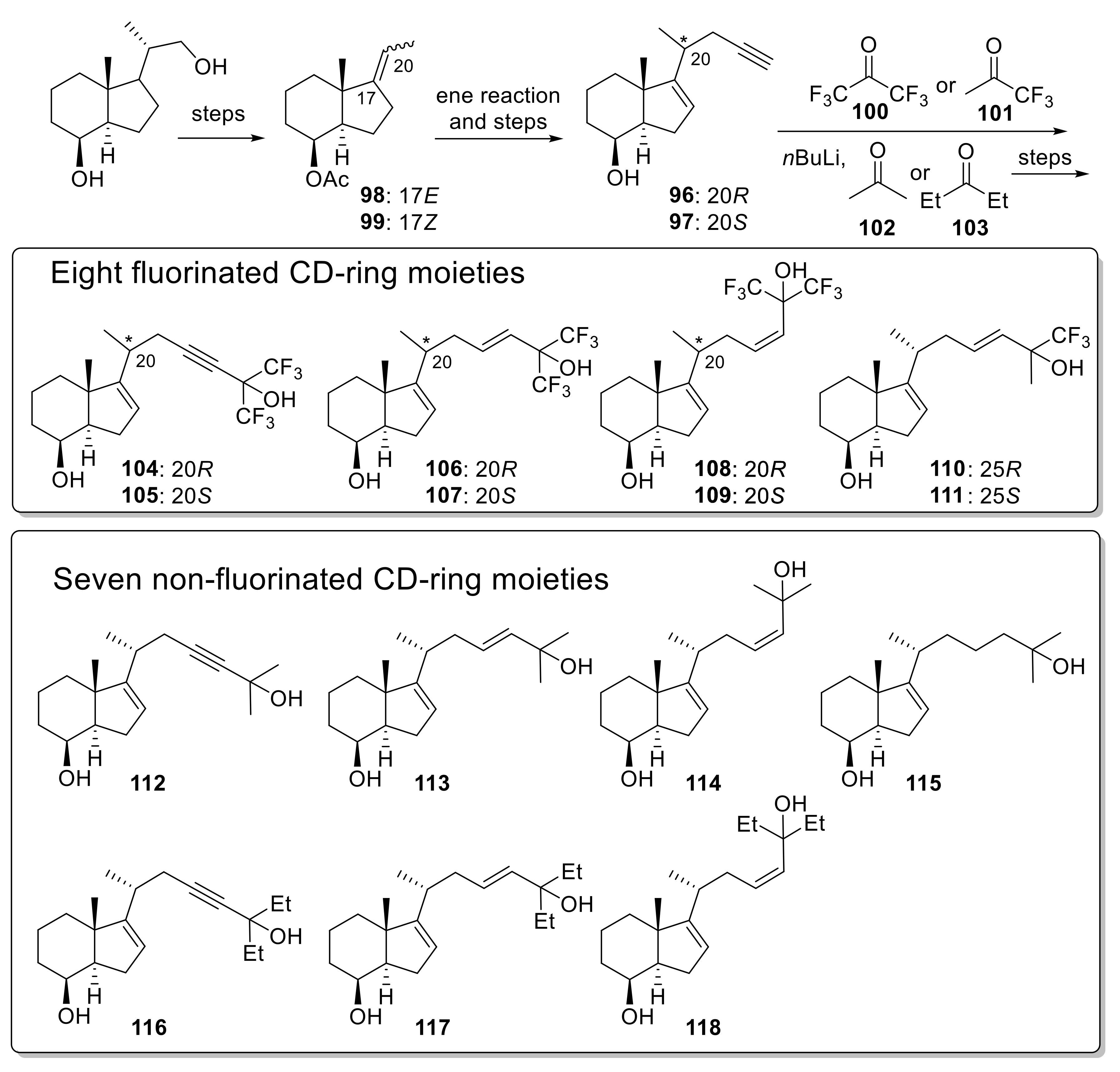
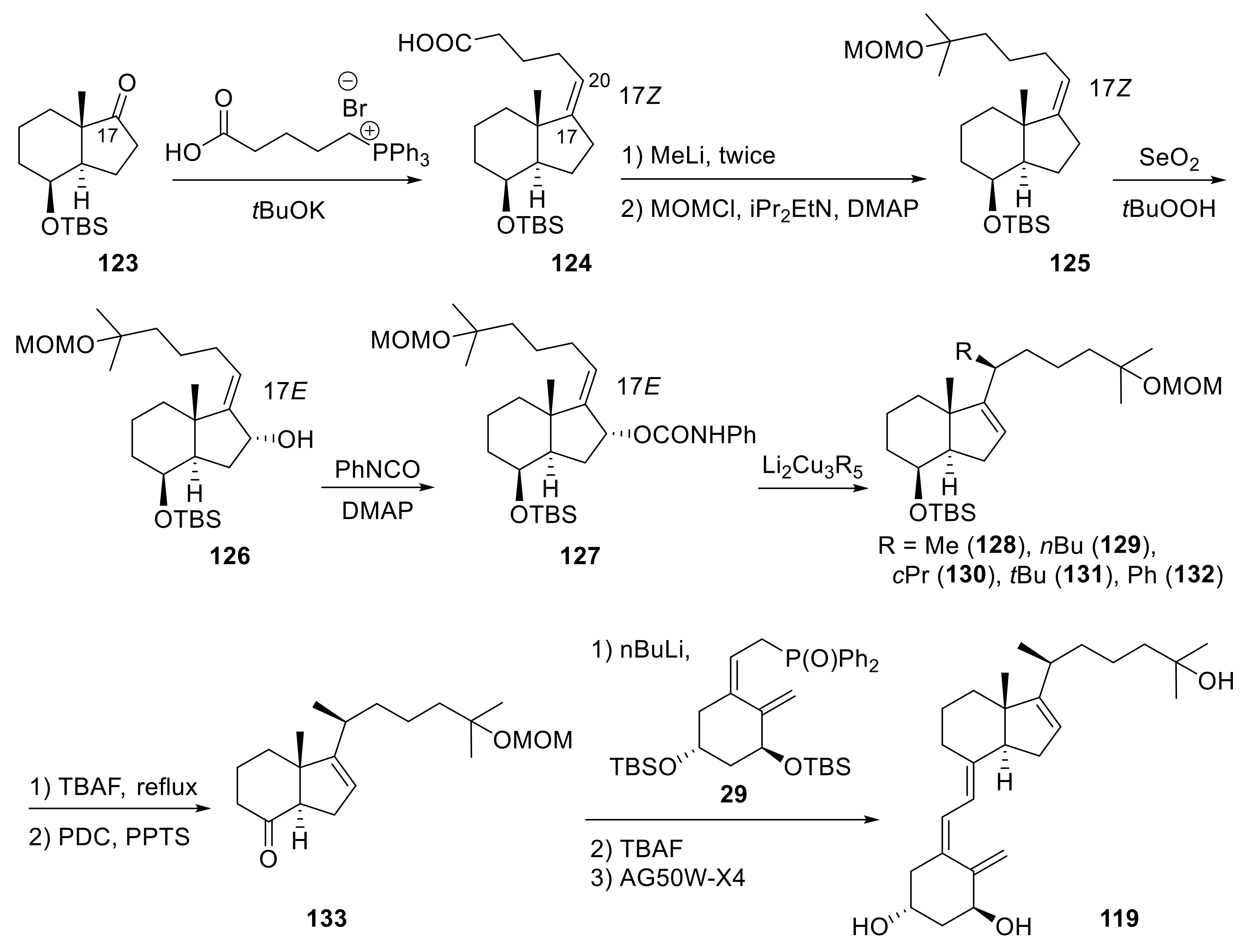



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawagoe, F.; Mototani, S.; Kittaka, A. The Synthesis and Biological Evaluation of D-Ring-Modified Vitamin D Analogues. Biomolecules 2021, 11, 1639. https://doi.org/10.3390/biom11111639
Kawagoe F, Mototani S, Kittaka A. The Synthesis and Biological Evaluation of D-Ring-Modified Vitamin D Analogues. Biomolecules. 2021; 11(11):1639. https://doi.org/10.3390/biom11111639
Chicago/Turabian StyleKawagoe, Fumihiro, Sayuri Mototani, and Atsushi Kittaka. 2021. "The Synthesis and Biological Evaluation of D-Ring-Modified Vitamin D Analogues" Biomolecules 11, no. 11: 1639. https://doi.org/10.3390/biom11111639





