Application of Pre-Column Labeling Liquid Chromatography for Canine Plasma-Free Amino Acid Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Diurnal Fluctuations in Canine PFAA Levels Measured with Pre-Column Labeling LC
| No. | Sex | Age (Years) | Body Weight (kg) |
|---|---|---|---|
| 1 | Male | 4 | 12 |
| 2 | Male | 11 | 11 |
| 3 | Male | 9 | 13 |
| 4 | Female | 9 | 11 |
| 5 | Female | 5 | 10 |
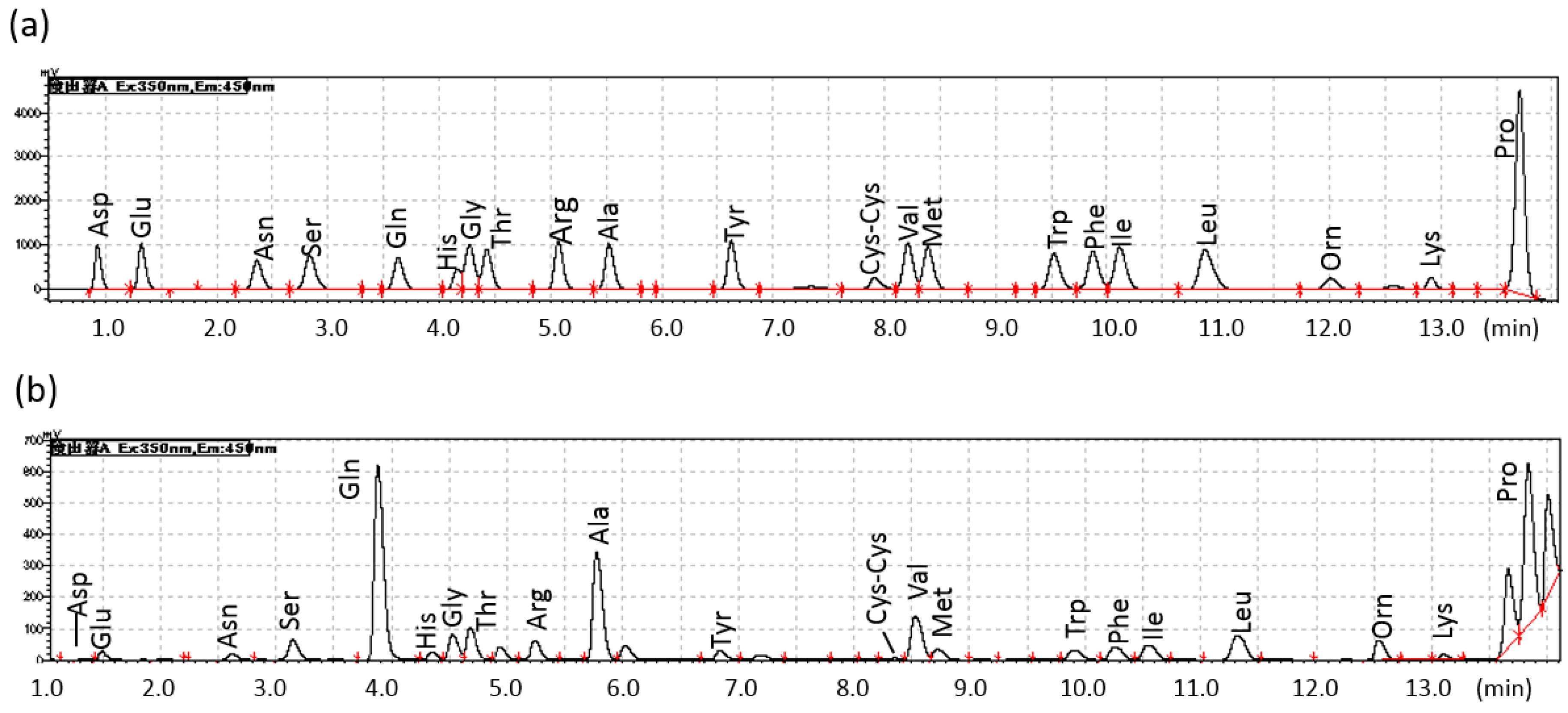

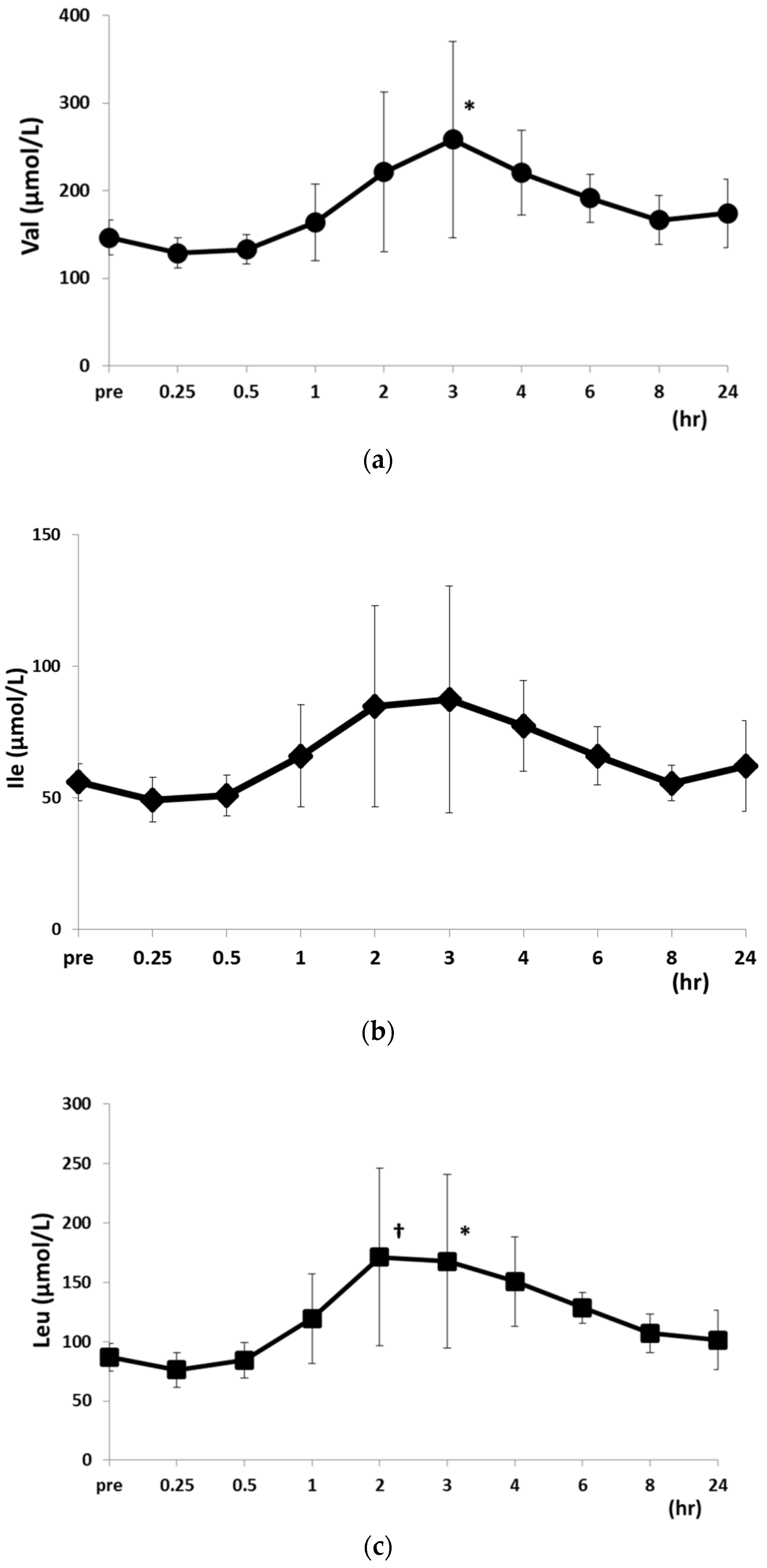
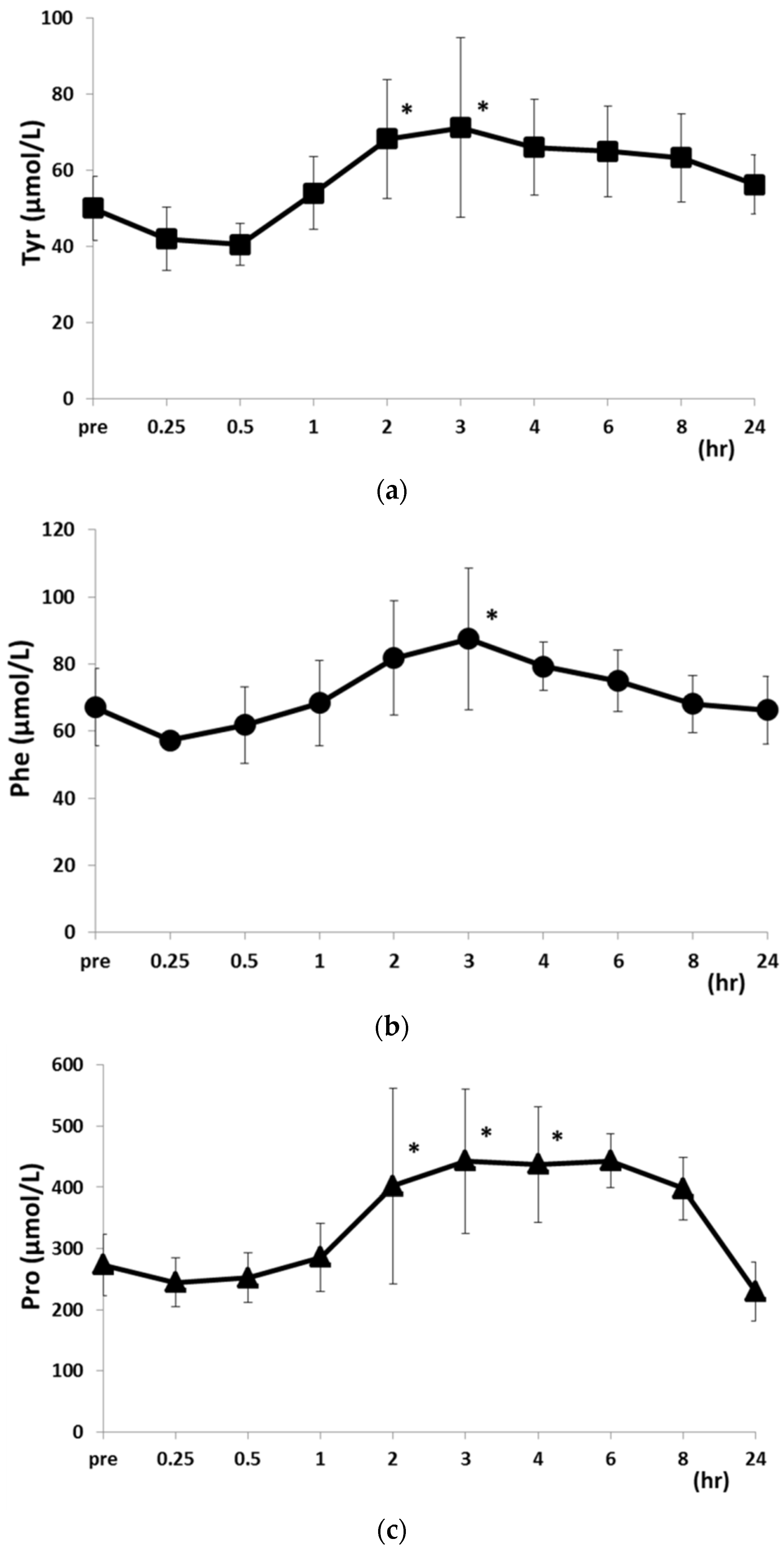
2.2. Changes in PFAA Levels after Food Intake
| Pre | 15 min | 30 min | 1 h | 2 h | |||
|---|---|---|---|---|---|---|---|
| His | 803.4 ± 88.4 | 713.6 ± 96.9 | 725.2 ± 85.3 | 747.4 ± 99.7 | 844.1 ± 184.6 | ||
| Thr | 66.1 ± 27.0 | 62.1 ± 25.4 | 63.9 ± 19.7 | 71.6 ± 10.2 | 96.6 ± 16.5 | ||
| Arg | 110.3 ± 21.0 | 95.7 ± 25.7 | 98.2 ± 21.1 | 106.5 ± 15.3 | 123.7 ± 34.8 | ||
| Val | 146.4 ± 19.8 | 128.8 ± 17.5 | 133.0 ± 16.6 | 164.1 ± 43.9 | 221.1 ± 91.2 | ||
| Met | 61.4 ± 7.4 | 50.6 ± 8.9 | 55.7 ± 7.1 | 58.4 ± 7.7 | 68.3 ± 15.3 | ||
| Trp | 79.7 ± 26.9 | 79.6 ± 28.7 | 88.1 ± 22.2 | 97.9 ± 17.1 | 111.6 ± 27.6 | ||
| Phe | 67.1 ± 11.5 | 57.3 ± 1.8 | 61.8 ± 11.4 | 68.3 ± 12.7 | 81.8 ± 16.9 | ||
| Ile | 56.1 ± 7.1 | 49.4 ± 8.3 | 51.0 ± 7.8 | 65.9 ± 19.4 | 84.8 ± 38.2 | ||
| Leu | 86.8 ± 11.4 | 76.1 ± 14.7 | 84.4 ± 14.9 | 119.5 ± 37.7 | 171.3 ± 74.6 | ||
| Lys | 51.3 ± 23.2 | 36.1 ± 7.3 | 35.8 ± 5.2 | 45.2 ± 18.4 | 52.6 ± 42.2 | ||
| Asp | 26.8 ± 4.9 | 22.9 ± 8.8 | 23.4 ± 3.9 | 22.8 ± 4.5 | 27.9 ± 11.2 | ||
| Glu | 80.6 ± 16.2 | 84.3 ± 8.8 | 74.5 ± 11.7 | 70.8 ± 5.0 | 79.2 ± 20.4 | ||
| Asn | 62.1 ± 6.0 | 67.6 ± 32.2 | 58.8 ± 12.9 | 74.3 ± 13.0 | 92.9 ± 27.0 | ||
| Ser | 214.9 ± 29.4 | 182.7 ± 12.3 | 201.5 ± 31.7 | 188.2 ± 16.7 | 207.5 ± 42.0 | ||
| Gln | 909.1 ± 137.1 | 779.7 ± 119.0 | 779.0 ± 105.6 | 752.1 ± 82.7 | 834.1 ± 205.9 | ||
| Gly | 157.2 ± 30.1 | 135.4 ± 25.2 | 143.2 ± 31.0 | 142.7 ± 23.4 | 159.3 ± 19.7 | ||
| Ala | 514.3 ± 55.1 | 452.9 ± 55.4 | 465.3 ± 70.3 | 490.1 ± 38.4 | 578.0 ± 160.0 | ||
| Tyr | 50.0 ± 8.5 | 42.0 ± 8.3 | 40.5 ± 5.6 | 54.0 ± 9.6 | 68.2 ± 15.6 | ||
| Cys | 17.7 ± 9.4 | 14.3 ± 7.4 | 12.5 ± 1.7 | 14.8 ± 1.7 | 17.5 ± 5.3 | ||
| Pro | 273.3 ± 50.3 | 244.4 ± 39.7 | 252.1 ± 40.9 | 285.7 ± 56.2 | 402.3 ± 160.1 | ||
| 3 h | 4 h | 6 h | 8 h | 24 h | |||
| His | 776.7 ± 139.2 | 687.7 ± 109.2 | 809.0 ± 111.2 | 807.9 ± 100.9 | 665.3 ± 116.2 | ||
| Thr | 92.8 ± 36.5 | 73.9 ± 24.4 | 67.2 ± 18.8 | 61.8 ± 17.8 | 57.7 ± 20.1 | ||
| Arg | 122.8 ± 37.9 | 108.9 ± 22.4 | 113.3 ± 24.5 | 110.9 ± 9.5 | 112.3 ± 20.6 | ||
| Val | 258.3 ± 20.60 * | 220.6 ± 48.1 * | 191.3 ± 27.4 | 166.5 ± 27.7 | 174.3 ± 39.0 | ||
| Met | 40.7 ± 24.5 | 54.8 ± 24.5 | 64.9 ± 7.8 | 63.9 ± 12.9 | 51.8 ± 12.9 | ||
| Trp | 104.2 ± 12.94 | 110.8 ± 12.97 | 111.1 ± 27.3 | 98.5 ± 26.5 | 52.4 ± 26.6 | ||
| Phe | 87.5 ± 21.1 * | 79.3 ± 7.2 * | 74.9 ± 9.1 | 68.1 ± 8.4 | 66.2 ± 10.1 | ||
| Ile | 87.3 ± 43.1 | 77.3 ± 43.1 | 66.0 ± 11.0 | 55.7 ± 6.9 | 62.3 ± 6.93 | ||
| Leu | 167.5 ± 69.3 * | 150.7 ± 69.8 * | 128.7 ± 13.0 | 107.1 ± 16.2 | 101.3 ± 16.2 | ||
| Lys | 86.3 ± 16.1 | 94.4 ± 16.3 | 102.6 ± 68.4 | 93.4 ± 52.6 | 78.4 ± 52.6 | ||
| Asp | 31.3 ± 10.6 | 25.1 ± 7.8 | 29.3 ± 10.3 | 32.8 ± 13.6 | 29.0 ± 13.6 | ||
| Glu | 91.5 ± 40.0 | 70.9 ± 41.5 | 79.4 ± 17.5 | 83.1 ± 14.8 | 85.1 ± 14.8 | ||
| Asn | 91.7 ± 14.8 | 93.1 ± 14.8 | 94.7 ± 24.4 | 84.5 ± 14.6 | 58.3 ± 14.8 | ||
| Ser | 215.9 ± 14.66 | 211.7 ± 14.62 | 240.4 ± 45.5 | 238.2 ± 31.4 | 186.5 ± 31.4 | ||
| Gln | 812.7 ± 31.46 | 817.8 ± 31.41 | 831.0 ± 94.6 | 814.8 ± 64.2 | 887.3 ± 95.8 | ||
| Gly | 160.0 ± 95.8 | 171.7 ± 95.8 | 185.8 ± 40.3 | 181.7 ± 30.3 | 139.9 ± 30.3 | ||
| Ala | 538.2 ± 130.5 | 485.8 ± 130.4 | 500.1 ± 41.6 | 458.4 ± 38.8 | 489.1 ± 38.8 | ||
| Tyr | 71.2 ± 38.8 | 66.0 ± 38.8 | 64.9 ± 12.0 | 63.2 ± 11.5 | 56.2 ± 11.7 | ||
| Cys | 18.5 ± 2.3 | 21.0 ± 11.5 | 16.9 ± 2.3 | 16.4 ± 4.3 | 19.8 ± 7.4 | ||
| Pro | 442.9 ± 117.7 | 437.1 ± 117.7 | 442.8 ± 44.0 | 398.0 ± 51.3 | 229.9 ± 51.3 | ||
3. Experimental Section
3.1. Animals
3.2. Sample Collection and Processing
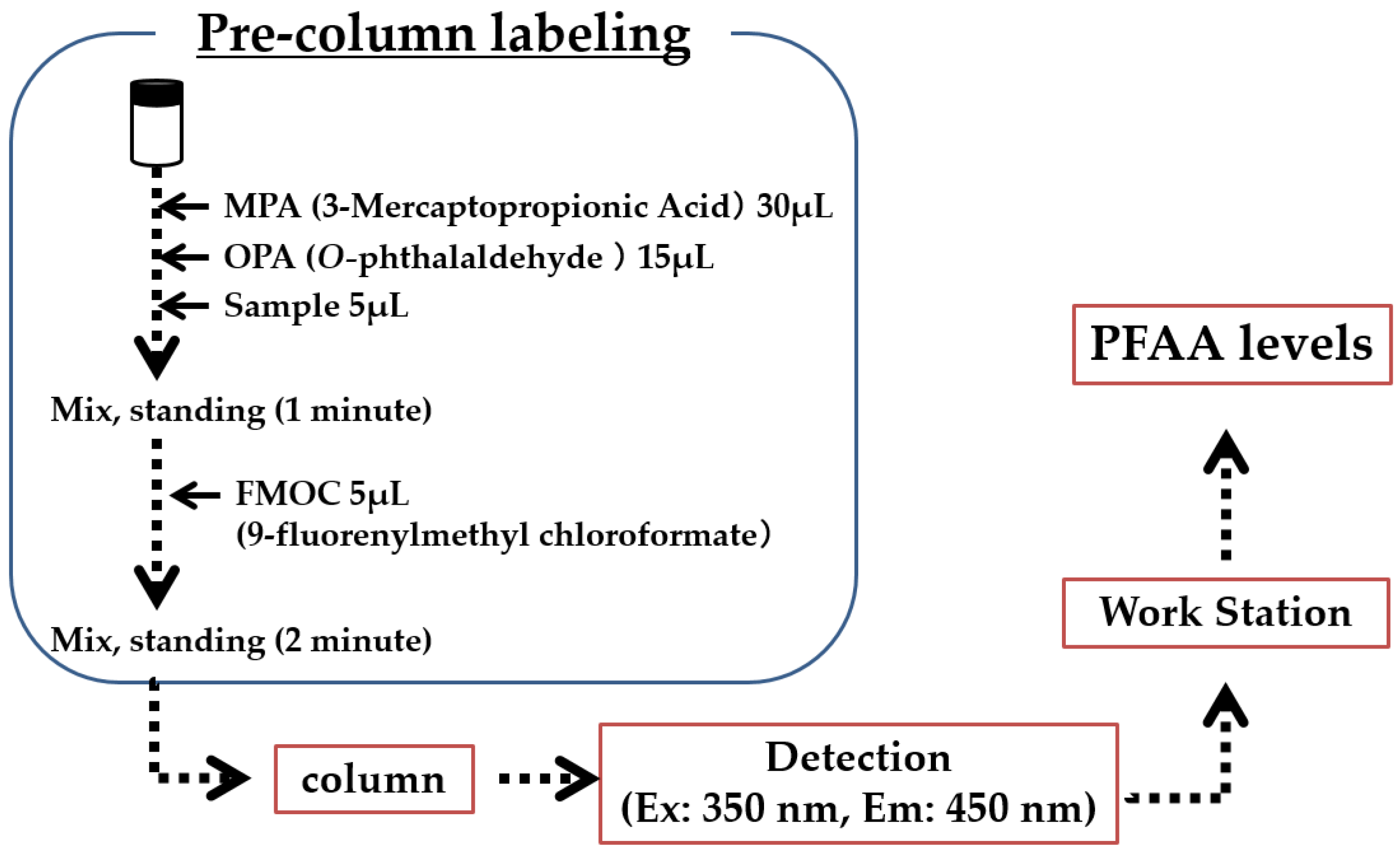
3.3. PFAA Measurements
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brosnan, J.T. Interorgan amino acid transport and its regulation. J. Nutr. 2003, 133, 2068S–2072S. [Google Scholar] [PubMed]
- Maeda, J.; Higashiyama, M.; Imaizumi, A.; Nakayama, T.; Yamamoto, H.; Daimon, T.; Yamakado, M.; Imamura, F.; Kodama, K. Possibility of multivariate function composed of plasma amino acid profiles as a novel screening index for non-small cell lung cancer: A case control study. BMC Cancer 2010. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, Y.; Higashiyama, M.; Gochi, A.; Akaike, M.; Ishikawa, T.; Miura, T.; Saruki, N.; Bando, E.; Kimura, H.; Imamura, F.; et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS ONE 2011, 6, e24143. [Google Scholar] [CrossRef] [PubMed]
- Proenza, A.M.; Oliver, J.; Palou, A.; Roca, P. Breast and lung cancer are associated with a decrease in blood cell amino acid content. J. Nutr. Biochem. 2003, 14, 133–138. [Google Scholar] [CrossRef]
- Fukutake, N.; Ueno, M.; Hiraoka, N.; Shimada, K.; Shiraishi, K.; Saruki, N.; Ito, T.; Yamakado, M.; Ono, N.; Imaizumi, A.; et al. A novel multivariate index for pancreatic cancer detection based on the plasma free amino acid profile. PLoS ONE 2015, 10, e0132223. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Tsuka, T.; Imagawa, T.; Minami, S.; Okamoto, Y. Plasma free amino acid profiles of canine mammary gland tumors. J. Vet. Sci. 2012, 13, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, M.; Baba, Y.; Tamai, R.; Yamamoto, R.; Komori, M.; Mori, T.; Takenaka, S. Profiling of plasma metabolites in canine oral melanoma using gas chromatography-mass spectrometry. J. Vet. Med. Sci. 2015, 77, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, H.; Dettmer, K.; Gronwald, W.; Oefner, P.J. Advances in amino acid analysis. Anal. Bioanal. Chem. 2009, 393, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Terrlink, T.; van Leeuwen, P.A.; Houdijk, A. Plasma amino acids determined by liquid chromatography within 17 minutes. Clin. Chem. 1994, 40, 245–249. [Google Scholar] [PubMed]
- Ashley, D.V.; Barclay, D.V.; Chauffard, F.A.; Moennoz, D.; Leathwood, P.D. Plasma amino acid responses in humans to evening meals of differing nutritional composition. Am. J. Clin. Nutr. 1982, 36, 143–153. [Google Scholar] [PubMed]
- Fernstrom, J.D.; Wurtman, R.J.; Hammarstrom-Wiklund, B.; Rand, W.M.; Munro, H.N.; Davidson, C.S. Diurnal variations in plasma concentrations of tryptophan, tyrosine, and other neutral amino acids: Effect of dietary protein intake. Am. J. Clin. Nutr. 1979, 32, 1912–1922. [Google Scholar] [PubMed]
- Nasset, E.S.; Heald, F.P.; Calloway, D.H.; Margen, S.; Schneeman, P. Amino acids in human blood plasma after single meals of meat, oil, sucrose and whiskey. J. Nutr. 1979, 109, 621–630. [Google Scholar] [PubMed]
- Van Loon, L.J.; Saris, W.H.; Verhagen, H.; Wagenmakers, A.J. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar] [PubMed]
- Chan, D.L.; Rozanski, E.A.; Freeman, L.M. Relationship among plasma amino acids, C-reactive protein, illness severity, and outcome in critically ill dogs. J. Vet. Intern. Med. 2009, 23, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kondo, K.; Tanaka, T.; Muramatsu, T.; Yoshida, H.; Imaizumi, A.; Nagao, K.; Noguchi, Y.; Miyano, H. Reference intervals for plasma-free amino acid in a Japanese population. Ann. Clin. Biochem. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Riggio, O.; Merli, M.; Pieche, U.; Romiti, A.; Pasqualetti, P.; Coppola, A.; Danese, D.; Cugini, P.; Capocaccia, L. Circadian rhythmicity of plasma amino acid variations in healthy subjects. Recent. Prog. Med. 1989, 80, 591–593. [Google Scholar]
- Tsai, P.; Wu, W.; Huong, P. Circadian variations in plasma neutral and basic amino acid concentrations in young men on an ordinary Taiwanese diet. J. Formos. Med. Assoc. 2000, 99, 151–157. [Google Scholar] [PubMed]
- Svanberg, G.K. Biological variations in serum total hydroxyproline concentration in the beagle dog. Lab. Anim. 1988, 22, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Takamori, Y.; Minami, S. Effects of oral glucosamine hydrochloride administration on plasma free amino acid concentrations in dogs. Mar. Drugs 2011, 9, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Imaizumi, A.; Ando, T.; Tochikubo, O. The overnight effect of dietary energy balance on postprandial plasma free amino acid (PFAA) profiles in Japanese adult men. PLoS ONE 2013, 8, e62929. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Colombani, P.C.; Langhans, W.; Wenk, C. Carbohydrate to protein ratio in food and cognitive performance in the morning. Physiol. Behav. 2002, 75, 411–423. [Google Scholar] [CrossRef]
- Lacroix, M.; Bos, C.; Leonil, J.; Airinei, G.; Luengo, C.; Daré, S.; Benamouzig, R.; Fouillet, H.; Fauquant, J.; Tomé, D.; et al. Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. Am. J. Clin. Nutr. 2006, 84, 1070–1079. [Google Scholar] [PubMed]
- Marrs, T.C.; Addison, J.M.; Burston, D.; Matthews, D.M. Changes in plasma amino acid concentrations in man after ingestion of an amino acid mixture simulating casein, and a tryptic hydrolysate of casein. Br. J. Nutr. 1975, 34, 259–265. [Google Scholar] [PubMed]
- Hutchison, A.T.; Piscitelli, D.; Horowitz, M.; Jones, K.L.; Clifton, P.M.; Standfield, S.; Hausken, T.; Feinle-Bisset, C.; Luscombe-Marsh, N.D. Acute load-dependent effects of oral whey protein on gastric emptying, gut hormone release, glycemia, appetite, and energy intake in healthy men. Am. J. Clin. Nutr. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Faller, D.V. Neutral amino acids in the brain: Changes in response to food ingestion. J. Neurochem. 1978, 30, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Sheffield-Moore, M.; Rasmussen, B.B.; Wolfe, R.R. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. J. Am. Med. Assoc. 2001, 286, 1206–1212. [Google Scholar] [CrossRef]
- Dossin, O.; Rupassara, S.I.; Weng, H.Y.; Williams, D.A.; Garlick, P.J.; Schoeman, J.P. Effect of parvoviral enteritis on plasma citrulline concentration in dogs. J. Vet. Intern. Med. 2011, 25, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Joyce, R.; Kuziene, V.; Zou, X.; Wang, X.; Pullen, F.; Loo, R.L. Development and validation of an ultra-performance liquid chromatography quadrupole time of flight mass spectrometry method for rapid quantification of free amino acids in human urine. Amino Acids 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; D’Alessandro, A.; Hansen, K.C. Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino Acids 2015, 47, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azuma, K.; Hirao, Y.; Hayakawa, Y.; Murahata, Y.; Osaki, T.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Ito, N. Application of Pre-Column Labeling Liquid Chromatography for Canine Plasma-Free Amino Acid Analysis. Metabolites 2016, 6, 3. https://doi.org/10.3390/metabo6010003
Azuma K, Hirao Y, Hayakawa Y, Murahata Y, Osaki T, Tsuka T, Imagawa T, Okamoto Y, Ito N. Application of Pre-Column Labeling Liquid Chromatography for Canine Plasma-Free Amino Acid Analysis. Metabolites. 2016; 6(1):3. https://doi.org/10.3390/metabo6010003
Chicago/Turabian StyleAzuma, Kazuo, Yoshiko Hirao, Yoshihiro Hayakawa, Yusuke Murahata, Tomohiro Osaki, Takeshi Tsuka, Tomohiro Imagawa, Yoshiharu Okamoto, and Norihiko Ito. 2016. "Application of Pre-Column Labeling Liquid Chromatography for Canine Plasma-Free Amino Acid Analysis" Metabolites 6, no. 1: 3. https://doi.org/10.3390/metabo6010003





