Safety Evaluation of Cosmetic Ingredients Regarding Their Skin Sensitization Potential
Abstract
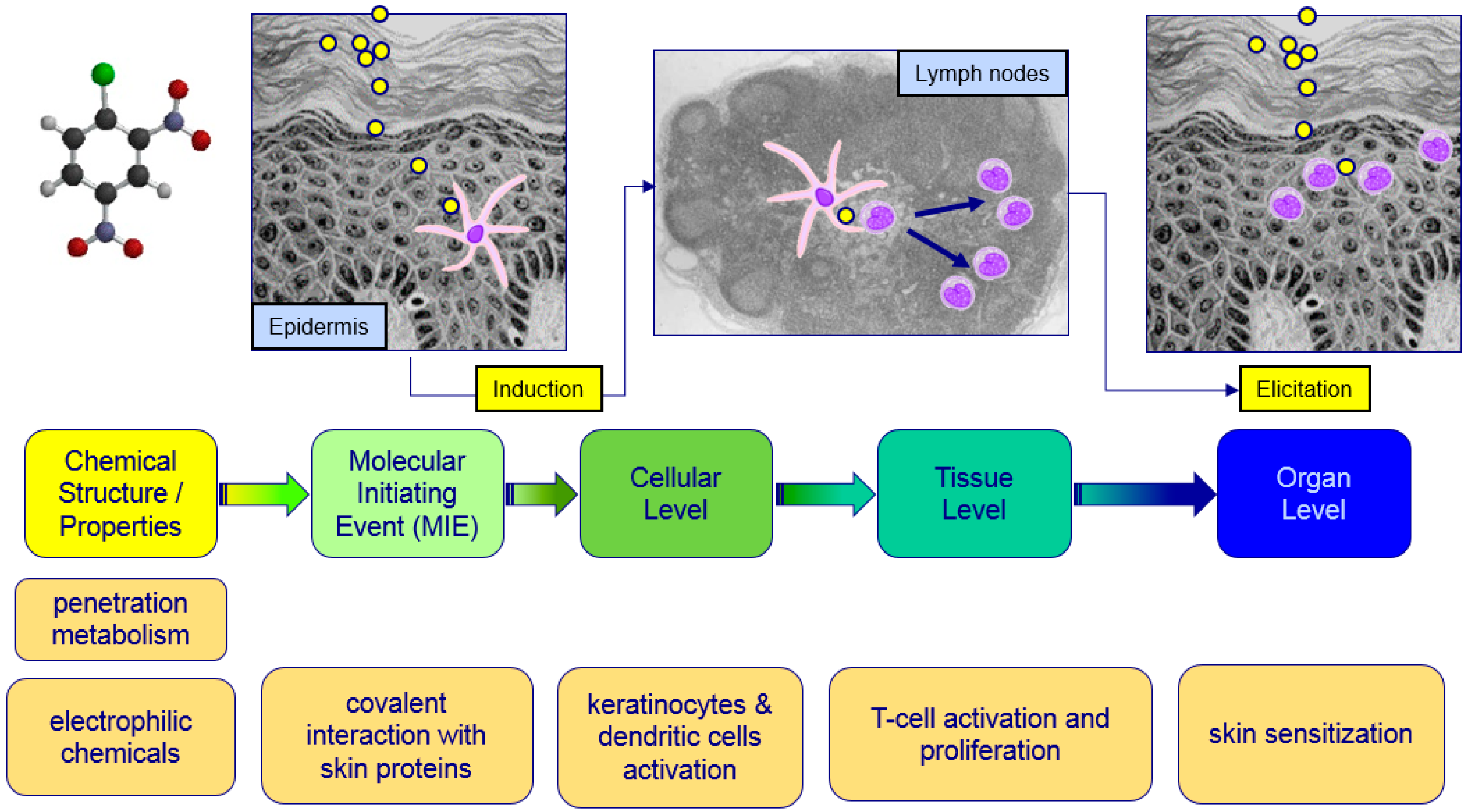
:1. Introduction
2. Regulatory Requirements for Chemicals
2.1. Data Obtained from Animal Testing
2.2. Data Obtained from New Testing Approach
2.3. Assessment of Sensitizing Potential Using A Weight of Evidence Approach
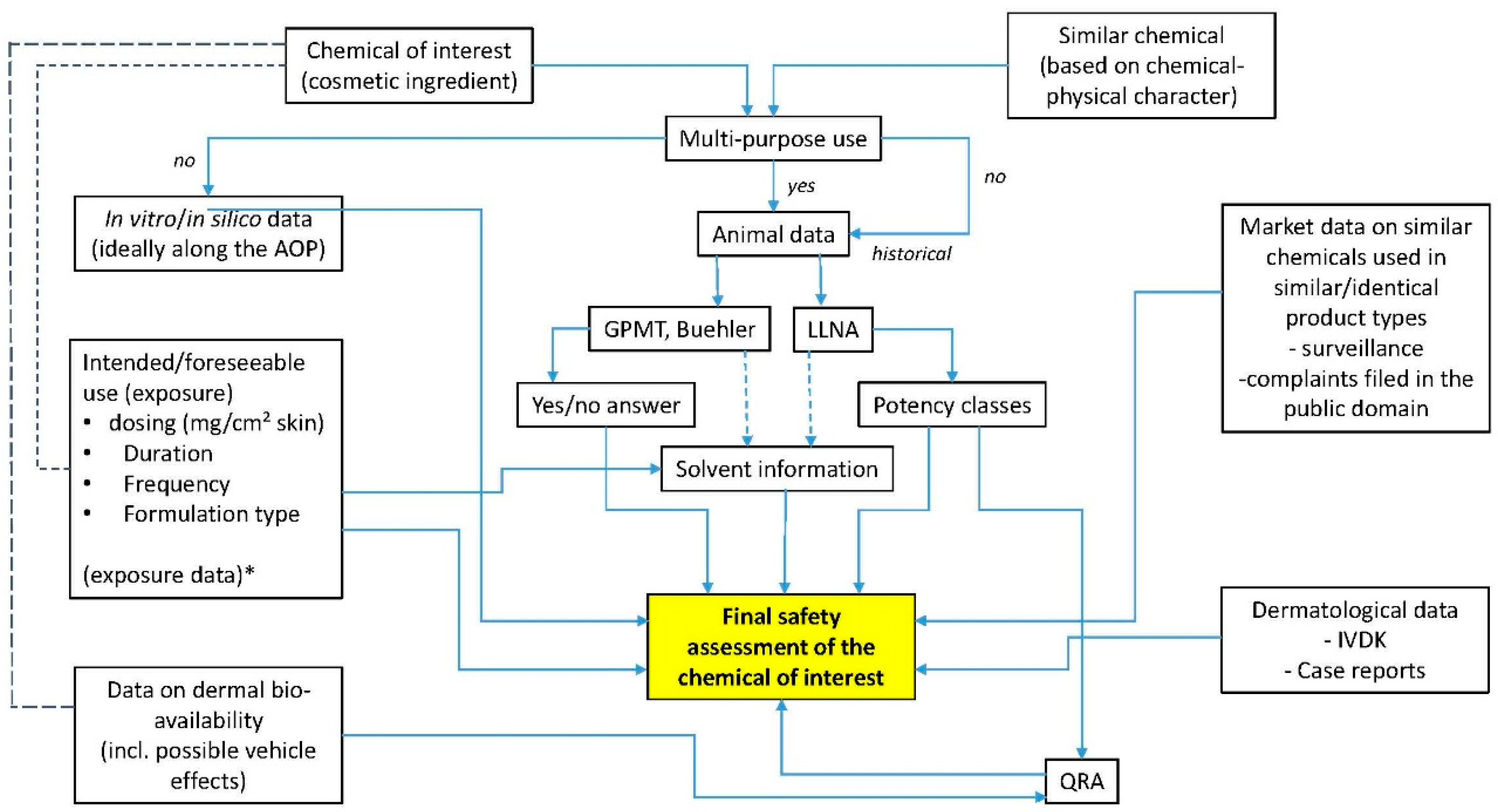
3. Product Safety Evaluation
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Steiling, W.; Vohr, H.W. Regulatory Assessment of Skin Sensitisation. In Dermal Absorption and Toxicity Assessment, Drugs and the Pharmaceutical Sciences, 2nd ed.; Roberts, M.S., Walters, K.A., Eds.; Informa Healthcare USA, Inc.: New York, NY, USA, 2008; Volume 177, pp. 523–535. [Google Scholar]
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Available online: http://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:32006R1907 (accessed on 24 March 2016).
- EU Directive 2000/33/EC of the European Parliament and of the Council of 25 April 2000 Adapting to Technical Progress for the 27th Time Council Directive 67/548/EEC on the Approximation of Laws, Regulations and Administrative Provisions Relating to the Classification, Packaging and Labelling of Dangerous Substances. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1458813012217&uri=CELEX:32000L0033 (accessed on 24 March 2016).
- ECHA. Guidance on the Application of the CLP Criteria, Guidance to Regulation (EC) No 1272/2008 on Classification, Labelling and Packaging (CLP) of Substances and Mixtures, Version 4.1; European Chemicals Agency (ECHA): Helsinki, Finland, 2015; pp. 328–357. [Google Scholar]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R1223 (accessed on 24 March 2016).
- Scientific Committee on Consumer Safety (SCCS). The SCCS’s Notes of Guidance for the Testing of Cosmetic Substances and Their Safety Evaluation; 9th Revision, SCCS/1564/15; Scientific Committee on Consumer Safety (SCCS): Luxembourg, Luxembourg, 2015. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). Guideline for Testing of Chemicals No. 406. Skin Sensitisation; Updated Guideline, adopted 17th July 1992; OECD: Paris, France, 1992. [Google Scholar]
- Steiling, W.; Basketter, D.; Berthold, K.; Butler, M.; Garrigue, J.-L.; Kimber, I.; Lea, L.; Newsome, C.; Roggeband, R.; Stropp, G.; et al. Skin Sensitisation Testing—New Perspectives and Recommendations. Food Chem. Toxicol. 2001, 39, 293–301. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). Guideline for Testing of Chemicals No. 429. Skin Sensitisation: Local Lymph Node Assay; Updated Guideline, adopted 24th April 2002; OECD: Paris, France, 2002. [Google Scholar]
- Steiling, W. PCS.01 Risk Assessment: A Brief History for ACD. Contact Dermat. 2004, 50, 122. [Google Scholar] [CrossRef]
- Gerberick, F.G.; Robinson, M.K.; Ryan, C.A.; Dearman, R.J.; Kimber, I.; Basketter, D.A.; Wright, Z.; Marks, J.G. Contact Allergenic Potency: Correlation of Human and Local Lymph Node Assay Data. Am. J. Contact Dermat. 2001, 2, 156–161. [Google Scholar]
- Basketter, D.A.; McFadden, J.F.; Gerberick, F.; Cockshott, A.; Kimber, I. Nothing is perfect, not even the local lymph node assay: A commentary and the implications for REACH. Contact Dermat. 2009, 60, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I.; Basketter, D.A.; Berthold, K.; Butler, M.; Garrigue, J.-L.; Lea, L.; Newsome, C.; Roggeband, R.; Steiling, W.; Stropp, G.; et al. Skin Sensitisation Testing in Potency and Risk Assessment. Toxicol. Sci. 2001, 59, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I.; Basketter, D.A.; Butler, M.; Gamer, A.; Garrigue, J.-L.; Gerberick, G.F.; Newsome, C.; Steiling, W.; Vohr, H.-W. Classification of Contact Allergens According to Potency: Proposals. Food Chem. Toxicol. 2003, 41, 1799–1809. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Guideline for Testing of Chemicals No. 442A. Skin Sensitisation: Local Lymph Node Assay: DA; OECD: Paris, France, 2010. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). Guideline for Testing of Chemicals No. 442B. Skin Sensitisation: Local Lymph Node Assay: BrdU-ELISA; OECD: Paris, France, 2010. [Google Scholar]
- Griem, P.; Goebel, C.; Scheffler, H. Proposal for a risk assessment methodology for skin sensitization based on sensitization potency data. Regul. Toxicol. Pharmacol. 2003, 38, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Gerberick, G.F.; Robinson, M.K.; Felter, S.; White, I.R.; Basketter, D. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermat. 2001, 45, 333–340. [Google Scholar] [CrossRef]
- Steiling, W. Potency of Skin Sensitisation—LLNA Data Used for a More Reliable Classification, Labelling and Risk Assessment. Toxicol. Lett. 2009, 189 (Suppl. 1), S26. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Products (SCCP). Memorandum Classification and Categorization of Skin Sensitisers and Grading of Test Reactions. Available online: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_s_01.pdf (accessed on 24 March 2016).
- EU Directive 2003/15/EC of the European Parliament and the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32003L0015 (accessed on 10 March 2016).
- Organisation for Economic Co-operation and Development (OECD). The Adverse Outcome Pathway for Skin Sensitization Initiated by Covalent Binding to Protein Part 1: Scientific Evidence; Series on Testing and Assessment No. 168; OECD: Paris, France, 2012. [Google Scholar]
- Tollefsen, K.E.; Scholz, S.; Cronin, M.T.; Edwards, S.W.; de Knecht, J.; Crofton, K.; Garcia-Reyero, N.; Hartung, T.; Worth, A.; Patlewicz, G. Applying Adverse Outcome Pathways (AOP) to support Integrated Approaches to Testing and Assessment (IATA). Regul. Pharmacol. Toxicol. 2014, 70, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Covalent Protein Binding Leading to Skin Sensitisation, Short Name: Skin Sensitisation AOP. Available online: https://aopwiki.org/wiki/index.php/Aop:40 (accessed on 10 March 2016).
- Reisinger, K.; Hoffmann, S.; Alépée, N.; Ashikaga, T.; Barroso, J.; Elcombe, C.; Gellatly, N.; Galbiati, V.; Gibbs, S.; Groux, H.; et al. Systematic evaluation of non-animal test methods for skin sensitisation safety assessment. Toxicol. In Vitro 2015, 29, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Ashikaga, T.; Yoshida, Y.; Hirota, M.; Yoneyama, K.; Itagaki, H.; Sakaguchi, H.; Miyazawa, M.; Ito, Y.; Suzuki, H.; Toyoda, H. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT), I. Optimization of the h-CLAT protocol. Toxicol. Vitro 2006, 20, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Ashikaga, T.; Miyazawa, M.; Yoshida, Y.; Ito, Y.; Yoneyama, K.; Hirota, M.; Itagaki, H.; Toyoda, H.; Suzuki, H. Development of an in vitro skin sensitization test using human cell lines: Human Cell Line Activation Test (h-CLAT), II. An inter-laboratory study of the h-CLAT. Toxicol. Vitro 2006, 20, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development (OECD). Guidelines for Testing of Chemicals No. 442C. In Chemico Skin Sensitisation: Direct Peptide Reactivity Assay; OECD: Paris, France, 2015. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). Guidelines for Testing of Chemicals No. 442D. In In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method; OECD: Paris, France, 2015. [Google Scholar]
- Urbisch, D.; Mehling, A.; Guth, K.; Ramirez, T.; Honarvar, N.; Kolle, S.; Landsiedel, R.; Jaworska, J.; Kern, P.S.; Gerberick, F.; et al. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul. Toxicol. Pharmacol. 2015, 71, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.; Ashikago, T.; Casati, S.; Hubesch, B.; Jaworska, J.; de Knecht, J.; Landsiedel, R.; Manou, I.; Mehling, A.; Petersohn, D.; et al. Alternatives for Skin Sensitisation: Hazard Identification and Potency Categorisation—Report from an EPAA/CEFIC LRI/Cosmetics Europe cross sector workshop, ECHA Helsinki, 23rd and 24th April 2015. Regul. Toxicol. Pharmacol. 2015, 73, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.A.; Alepee, N.; Ashikaga, T.; Barroso, J.; Gilmour, N.; Goebel, C.; Hibatallah, J.; Hoffmann, S.; Kern, P.; Martinozzi-Teissier, S.; et al. Categorisation of chemicals according to their relative human skin sensitizing potency. Dermatitis 2014, 25, 11–21. [Google Scholar] [CrossRef] [PubMed]
- OECD. Development of Integrated Approaches to Testing and Assessment at OECD. Joint Cefic LRI/Cosmetics Europe/EPAA workshop, 23–24 April 2015. Available online: http://cefic-lri.org/wp-content/uploads/2014/03/B_IATA-development-at-OECD-Compatibility-Mode.pdf (accessed on 10 March 2016).
- Scientific Committee on Consumer Safety (SCCS). Memorandum on Use of Human Data in Risk Assessment of Skin Sensitization (SCCS/1567/15); Scientific Committee on Consumer Safety (SCCS): Luxembourg, Luxembourg, 2015. [Google Scholar]
- EU Directive 1999/45/EC of the European Parliament and of the Council of 31 May 1999 concerning the approximation of laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:1999:166:TOC (accessed on 10 March 2016).
- Loveless, S.; Api, A.M.; Crevel, R.; Debruyne, E.; Gamer, A.; Jowsey, I.; Kern, P.; Kimber, I.; Lea, L.; Llloyd, P.; et al. Potency Values from the Local Lymph Node Assay: Application to Classification, Labelling and Risk Assessment. Regul. Pharmacol. Toxicol. 2010, 56, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.; Safford, B. Skin sensitization quantitative risk assessment: A review of underlying assumptions. Regul. Toxicol. Pharmacol. 2016, 74, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Basketter, D.A.; Cadby, P.A.; Cano, M.F.; Ellis, G.; Gerberick, G.F.; Griem, P.; McNamee, P.M.; Ryan, C.A.; Safford, R. Dermal sensitization quantitative risk assessment (QRA) for fragrance ingredients. Regul. Toxicol. Pharmacol. 2008, 52, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Felter, S.P.; Robinson, M.K.; Basketter, D.A.; Gerberick, G.F. A review of the scientific basis for uncertainty factors for use in quantitative risk assessment for the induction of allergic contact dermatitis. Contact Dermat. 2002, 47, 257–266. [Google Scholar] [CrossRef]
- IDEA-project (International Dialogue for the Evaluation of Allergens). Available online: http://www.ideaproject.info/about-idea (accessed on 10 March 2016).
- Kimber, I.; Dearman, R.J.; Basketter, D.A.; Ryan, C.A.; Gerberick, G.F.; McNamee, P.M.; Lalko, J.; Api, A.M. Dose metrics in the acquisition of skin sensitization: Thresholds and importance of dose per unit area. Regul. Toxicol. Pharmacol. 2008, 52, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.A.; Gerberick, G.F.; Kimber, I. Skin sensitisation, vehicle effects and the local lymph node assay. Food Chem. Toxicol. 2001, 39, 621–627. [Google Scholar] [CrossRef]
- Basketter, D.; Alepee, N.; Casati, S.; Crozier, J.; Eigler, D.; Griem, P.; Hubesch, B.; de Knecht, J.; Landsiedel, R.; Louekari, K.; et al. Skin sensitisation—Moving forward with non-animal strategies. Regul. Toxicol. Pharmacol. 2013, 67, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Steiling, W.; Ghosh, R.; Knübel, G.; Ryjkina, E. SAR meets LLNA—Structure Activity Relationship Triggers the Local Lymph Node Assay for Testing the Skin Sensitisation Potential. ALTEX 2005, 22, 273. [Google Scholar]
- Karlberg, A.-T.; Bergström, M.A.; Börje, A.; Luthman, K.; Nilsson, J.L.G. Allergic Contact Dermatitis—Formation, Structural Requirements, and Reactivity of Skin Sensitizers. Chem. Res. Toxicol. 2008, 21, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.; Zang, Q.; Kleinstreuer, N.; Paris, M.; Lehmann, D.M.; Choksi, N.; Matheson, J.; Jacobs, A.; Lowit, A.; Allen, D.; et al. Integrated decision strategies for skin sensitization hazard. J. Appl. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
| DPD | CLP | ECETOC Proposal | |||||
|---|---|---|---|---|---|---|---|
| threshold concentration of sensitizing ingredient [%] | ≥1.0 | ≥0.1 | ≥1.0 | ≥0.003 | ≥0.1 | ≥1.0 | ≥3.0 |
| potency of the sensitizing ingredient EC3 [%] | – | Cat 1A (≤2.0) | Cat 1B (>2.0) | extreme (<0.1) | strong (≥0.1, <1.0) | moderate (≥1.0, <10.0) | weak (≥10.0) |
| classification/labelling | R43/H317 | H317 | H317 | H317 | H317 | H317 | H317 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steiling, W. Safety Evaluation of Cosmetic Ingredients Regarding Their Skin Sensitization Potential. Cosmetics 2016, 3, 14. https://doi.org/10.3390/cosmetics3020014
Steiling W. Safety Evaluation of Cosmetic Ingredients Regarding Their Skin Sensitization Potential. Cosmetics. 2016; 3(2):14. https://doi.org/10.3390/cosmetics3020014
Chicago/Turabian StyleSteiling, Winfried. 2016. "Safety Evaluation of Cosmetic Ingredients Regarding Their Skin Sensitization Potential" Cosmetics 3, no. 2: 14. https://doi.org/10.3390/cosmetics3020014






