Plant Products for Innovative Biomaterials in Dentistry
Abstract
:1. Introduction
- oral rehabilitation in implantology (titanium implants)
- periodontal tissue regeneration.
2. Plant Product-Based Titanium Implant Coatings
| Plant products | Biomaterials | Applications | References |
|---|---|---|---|
| Malus domestica L. | Titanium implant coating | Dental implantology | In vitro: [12] In vivo (rats): [13] |
| Cissus quadrangularis L. | Periodontal filler in association with hydroxyapatite | Periodontal regeneration | Clinical trial: [14] |
| Carthamus tinctorius L | Periodontal filler in association with collagen sponge Periodontal filler in association with polylactide glycolic acid bioresorbable barrier | Periodontal regeneration Periodontal regeneration | In vivo (beagle dogs): [15] In vivo (beagle dogs): [16] |
| Glycine max L. | Bone filler | Alveolar bone regeneration | In vivo (rabbits): [17] |
2.1. Pectins

3. Plant Product-Based in Periodontal Regenerative Therapy
3.1. Cissus quadrangularis L.

3.2. Carthamus tinctorius L.
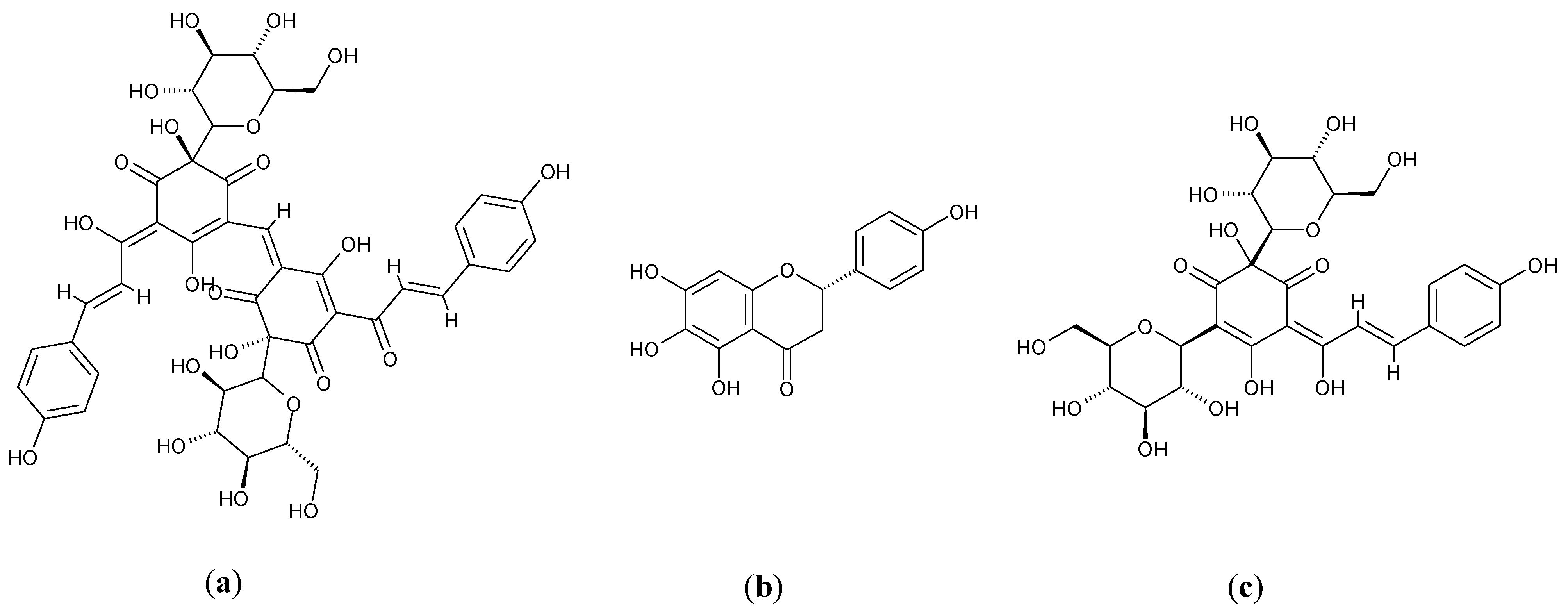
3.3. Glycine max L.

4. Conclusions
Acknowledgments
References
- Avila, G.; Misch, K.; Galindo-Moreno, P.; Wang, H.-L. Implant surface treatment using biomimetic agents. Implant Dent. 2009, 18, 17–26. [Google Scholar] [CrossRef]
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; García, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Brunski, J.B. In vivo bone response to biomechanical loading at the bone/dental-implant interface. Adv. Dent. Res. 1999, 13, 99–119. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, S172–S184. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brunski, J.; Wennerberg, A. “A requiem for the periodontal ligament” revisited. Int. J. Prosthodont. 2009, 22, 120–122. [Google Scholar]
- Clarke, S.A.; Revell, P.A. Integrin expression at the bone/biomaterial interface. J. Biomed. Mater. Res. 2001, 57, 84–91. [Google Scholar] [CrossRef]
- Kellar, R.S.; Kleinert, L.B.; Williams, S.K. Characterization of angiogenesis and inflammation surrounding ePTFE implanted on the epicardium. J. Biomed. Mater. Res. 2002, 61, 226–233. [Google Scholar] [CrossRef]
- Roberts, W.E. Bone tissue interface. J. Dent. Educ. 1988, 52, 804–809. [Google Scholar]
- Morra, M. Biochemical modification of titanium surfaces: Peptides and ECM proteins. Eur. Cell Mater. 2006, 12, 1–15. [Google Scholar]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, S185–S206. [Google Scholar] [CrossRef]
- Kokkonen, H.; Cassinelli, C.; Verhoef, R.; Morra, M.; Schols, H.A.; Tuukkanen, J. Differentiation of osteoblasts on pectin-coated titanium. Biomacromolecules 2008, 9, 2369–2376. [Google Scholar] [CrossRef]
- Kokkonen, H.; Niiranen, H.; Schols, H.A.; Morra, M.; Stenbäck, F.; Tuukkanen, J. Pectin-coated titanium implants are well-tolerated in vivo. J. Biomed. Mater. Res. A 2010, 93, 1404–1409. [Google Scholar]
- Jain, A.; Dixit, J.; Prakash, D. Modulatory effects of Cissus quadrangularis on periodontal regeneration by bovine-derived hydroxyapatite in intrabony defects: Exploratory clinical trial. J. Int. Acad. Periodontol. 2008, 10, 59–65. [Google Scholar]
- Kim, H.-Y.; Kim, C.-S.; Jhon, G.-J.; Moon, I.-S.; Choi, S.-H.; Cho, K.-S.; Chai, J.-K.; Kim, C.-K. The effect of safflower seed extract on periodontal healing of 1-wall intrabony defects in beagle dogs. J. Periodontol. 2002, 73, 1457–1466. [Google Scholar] [CrossRef]
- Song, W.-S.; Kim, C.-S.; Choi, S.-H.; Jhon, G.-J.; Kim, H.-Y.; Cho, K.-S.; Kim, C.-K.; Chai, J.-K. The effects of a bioabsorbable barrier membrane containing safflower seed extracts on periodontal healing of 1-wall intrabony defects in beagle dogs. J. Periodontol. 2005, 76, 22–33. [Google Scholar] [CrossRef]
- Merolli, A.; Nicolais, L.; Ambrosio, L.; Santin, M. A degradable soybean-based biomaterial used effectively as a bone filler in vivo in a rabbit. Biomed. Mater. 2010, 5. [Google Scholar]
- Yapo, B.M. Pineapple and banana pectins comprise fewer homogalacturonan building blocks with a smaller degree of polymerization as compared with yellow passion fruit and lemon pectins: Implication for gelling properties. Biomacromolecules 2009, 10, 717–721. [Google Scholar] [CrossRef]
- Redondo-Nevado, J.; Moyano, E.; Medina-Escobar, N.; Caballero, J.L.; Muñoz-Blanco, J. A fruit-specific and developmentally regulated endopolygalacturonase gene from strawberry (Fragaria × ananassa cv. Chandler). J. Exp. Bot. 2001, 52, 1941–1945. [Google Scholar] [CrossRef]
- Vincken, J.-P.; Schols, H.A.; Oomen, R.J.F.J.; McCann, M.C.; Ulvskov, P.; Voragen, A.G.J.; Visser, R.G.F. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol. 2003, 132, 1781–1789. [Google Scholar] [CrossRef]
- Bonnin, E.; Dolo, E.; Le Goff, A.; Thibault, J.-F. Characterisation of pectin subunits released by an optimised combination of enzymes. Carbohydr. Res. 2002, 337, 1687–1696. [Google Scholar] [CrossRef]
- Liu, L.; Won, Y.J.; Cooke, P.H.; Coffin, D.R.; Fishman, M.L.; Hicks, K.B.; Ma, P.X. Pectin/poly(lactide-co-glycolide) composite matrices for biomedical applications. Biomaterials 2004, 25, 3201–3210. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef]
- Munarin, F.; Giuliano, L.; Bozzini, S.; Tanzi, M.C.; Petrini, P. Mineral phase deposition on pectin microspheres. Mat. Sci. Eng. C 2010, 30, 491–496. [Google Scholar] [CrossRef]
- Munarin, F.; Petrini, P.; Tanzi, M.C.; Barbosa, M.A.; Granja, P.L. Biofunctional chemically modified pectin for cell delivery. Soft Matter 2012, 8, 4731–4739. [Google Scholar]
- Mishra, R.K.; Datt, M.; Pal, K.; Banthia, A.K. Preparation and characterization of amidated pectin based hydrogels for drug delivery system. J. Mater. Sci. Mater. Med. 2008, 19, 2275–2280. [Google Scholar] [CrossRef]
- Munarin, F.; Petrini, P.; Farè, S.; Tanzi, M.C. Structural properties of polysaccharide-based microcapsules for soft tissue regeneration. J. Mater. Sci. Mater. Med. 2010, 21, 365–375. [Google Scholar] [CrossRef]
- Ishii, T.; Matsunaga, T. Pectic polysaccharide rhamnogalacturonan II is covalently linked to homogalacturonan. Phytochemistry 2001, 57, 969–974. [Google Scholar]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Schols, H.A.; Voragen, A.G.; Colquhoun, I.J. Isolation and characterization of rhamnogalacturonan oligomers, liberated during degradation of pectic hairy regions by rhamnogalacturonase. Carbohydr. Res. 1994, 256, 97–111. [Google Scholar] [CrossRef]
- Schols, H.A.; Vierhuis, E.; Bakx, E.J.; Voragen, A.G. Different populations of pectic hairy regions occur in apple cell walls. Carbohydr. Res. 1995, 275, 343–360. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Cascardo, G.; Nagel, M.-D.; Della Volpe, C.; Siboni, S.; Maniglio, D.; Brugnara, M.; Ceccone, G.; Schols, H.A.; et al. Effects on interfacial properties and cell adhesion of surface modification by pectic hairy regions. Biomacromolecules 2004, 5, 2094–2104. [Google Scholar] [CrossRef]
- Nagel, M.-D.; Verhoef, R.; Schols, H.; Morra, M.; Knox, J.P.; Ceccone, G.; Della Volpe, C.; Vigneron, P.; Bussy, C.; Gallet, M.; et al. Enzymatically-tailored pectins differentially influence the morphology, adhesion, cell cycle progression and survival of fibroblasts. Biochim. Biophys. Acta 2008, 1780, 995–1003. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Chen, C.-H.; Sheu, M.-T.; Chen, T.-F.; Wang, Y.-C.; Hou, W.-C.; Liu, D.-Z.; Chung, T.-C.; Liang, Y.-C. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem. Pharmacol. 2006, 72, 1001–1009. [Google Scholar]
- Salman, H.; Bergman, M.; Djaldetti, M.; Orlin, J.; Bessler, H. Citrus pectin affects cytokine production by human peripheral blood mononuclear cells. Biomed. Pharmacother. 2008, 62, 579–582. [Google Scholar] [CrossRef]
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohyd. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Wang, N.L.; Kiyohara, H.; Matsumoto, T.; Otsuka, H.; Hirano, M.; Yamada, H. Polyclonal antibody against a complement-activating pectin from the roots of Angelica acutiloba. Planta Med. 1994, 60, 425–429. [Google Scholar] [CrossRef]
- Sakurai, M.H.; Matsumoto, T.; Kiyohara, H.; Yamada, H. B-cell proliferation activity of pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L. and its structural requirement. Immunology 1999, 97, 540–547. [Google Scholar] [CrossRef]
- Michaelsen, T.E.; Gilje, A.; Samuelsen, A.B.; Høgåsen, K.; Paulsen, B.S. Interaction between human complement and a pectin type polysaccharide fraction, PMII, from the leaves of Plantagomajor L. Scand. J. Immunol. 2000, 52, 483–490. [Google Scholar] [CrossRef]
- Dourado, F.; Madureira, P.; Carvalho, V.; Coelho, R.; Coimbra, M.A.; Vilanova, M.; Mota, M.; Gama, F.M. Purification, structure and immunobiological activity of an arabinan-rich pectic polysaccharide from the cell walls of Prunus dulcis seeds. Carbohydr. Res. 2004, 339, 2555–2566. [Google Scholar] [CrossRef]
- Wang, X.-S.; Dong, Q.; Zuo, J.-P.; Fang, J.-N. Structure and potential immunological activity of apectin from Centellaasiatica (L.) Urban. Carbohydr. Res. 2003, 338, 2393–2402. [Google Scholar] [CrossRef]
- Bussy, C.; Verhoef, R.; Haeger, A.; Morra, M.; Duval, J.-L.; Vigneron, P.; Bensoussan, A.; Velzenberger, E.; Cascardo, G.; Cassinelli, C.; et al. Modulating in vitro bone cell and macrophage behavior by immobilized enzymatically tailored pectins. J. Biomed. Mater. Res. A 2008, 86, 597–606. [Google Scholar]
- Kokkonen, H.E.; Ilvesaro, J.M.; Morra, M.; Schols, H.A.; Tuukkanen, J. Effect of modified pectin molecules on the growth of bone cells. Biomacromolecules 2007, 8, 509–515. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Elder, S.H.; Jouett, R.; Yang, Y.; Ong, J.L. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J. Biomater. Sci. Polym. Ed. 2003, 14, 1401–1409. [Google Scholar] [CrossRef]
- Nagel, M.-D.; Verhoef, R.; Schols, H.; Morra, M.; Knox, J.P.; Ceccone, G.; Della Volpe, C.; Vigneron, P.; Bussy, C.; Gallet, M.; et al. Enzymatically-tailored pectins differentially influence the morphology, adhesion, cell cycle progression and survival of fibroblasts. Biochim. Biophys. Acta 2008, 1780, 995–1003. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials—Biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R. Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J. Clin. Periodontol. 2008, 35, 117–135. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2010, 7, S93–S105. [Google Scholar] [CrossRef]
- Grzesik, W.J.; Narayanan, A.S. Cementum and periodontal wound healing and regeneration. Crit. Rev. Oral Biol. Med. 2002, 13, 474–484. [Google Scholar] [CrossRef]
- Taba, M., Jr.; Jin, Q.; Sugai, J.V.; Giannobile, W.V. Current concepts in periodontal bioengineering. Orthod. Craniofac. Res. 2005, 8, 292–302. [Google Scholar] [CrossRef]
- Benatti, B.B.; Silvério, K.G.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Physiological features of periodontal regeneration and approaches for periodontal tissue engineering utilizing periodontal ligament cells. J. Biosci. Bioeng. 2007, 103, 1–6. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lodi, G.; Sardella, A.; Carrassi, A.; Iriti, M. Plant polyphenols and oral health: Old phytochemicals for new fields. Curr. Med. Chem. 2012, 19, 1706–1720. [Google Scholar] [CrossRef]
- Srivastava, K.A.; Mishra, J.N.; Behera, B.R.; Shrivastava, A.K.; Srivastava, P.; Tiwari, B.N. A plant (Cissus quadrangularis) with various ethnopharmacological action: A review. J. Pharm. Res. 2011, 4, 1887–1890. [Google Scholar]
- Deka, D.K.; Lahon, L.C.; Saikia, J.; Mukit, A. Effect of Cissus quadrangularis in accelerating healing process of experimentally fractured radius-ulna of dog, a preliminary study. Indian J. Pharmacol. 1994, 26, 44–45. [Google Scholar]
- Mehta, M.; Kaur, N.; Bhutani, K.K. Determination of marker constituents from Cissus quadrangularis Linn. and their quantitation by HPTLC and HPLC. Phytochem. Anal. 2001, 12, 91–95. [Google Scholar] [CrossRef]
- Thakur, A.; Jain, V.; Hingorani, L.; Laddha, K.S. Phytochemical Studies on Cissus quadrangularis Linn. Pharmacogn. Res. 2009, 1, 213. [Google Scholar]
- Panthong, A.; Supraditaporn, W.; Kanjanapothi, D.; Taesotikul, T.; Reutrakul, V. Analgesic, anti-inflammatory and venotonic effects of Cissus quadrangularis Linn. J. Ethnopharmacol. 2007, 110, 264–270. [Google Scholar] [CrossRef]
- Jainu, M.; Mohan, K.V. Protective role of ascorbic acid isolated from Cissus quadrangularis on NSAID induced toxicity through immunomodulating response and growth factors expression. Int. Immunopharmacol. 2008, 8, 1721–1727. [Google Scholar] [CrossRef]
- Srisook, K.; Palachot, M.; Mongkol, N.; Srisook, E.; Sarapusit, S. Anti-inflammatory effect of ethyl acetate extract from Cissus quadrangularis Linn may be involved with induction of heme oxygenase-1 and suppression of NF-κB activation. J. Ethnopharmacol. 2011, 133, 1008–1014. [Google Scholar] [CrossRef]
- Potu, B.K.; Bhat, K.M.R.; Rao, M.S.; Nampurath, G.K.; Chamallamudi, M.R.; Nayak, S.R.; Muttigi, M.S. Petroleum ether extract of Cissus quadrangularis (Linn.) enhances bone marrow mesenchymal stem cell proliferation and facilitates osteoblastogenesis. Clinics (Sao Paulo) 2009, 64, 993–998. [Google Scholar] [CrossRef]
- Potu, B.K.; Rao, M.S.; Nampurath, G.K.; Chamallamudi, M.R.; Nayak, S.R.; Thomas, H. Anti-osteoporotic activity of the petroleum ether extract of Cissus quadrangularis Linn. in ovariectomized Wistar rats. Chang Gung Med. J. 2010, 33, 252–257. [Google Scholar]
- Potu, B.K.; Nampurath, G.K.; Rao, M.S.; Bhat, K.M.R. Effect of Cissus quadrangularis Linn on the development of osteopenia induced by ovariectomy in rats. Clin. Ter. 2011, 162, 307–312. [Google Scholar]
- Parisuthiman, D.; Singhatanadgit, W.; Dechatiwongse, T.; Koontongkaew, S. Cissus quadrangularis extract enhances biomineralization through up-regulation of MAPK-dependent alkaline phosphatase activity in osteoblasts. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 194–200. [Google Scholar] [CrossRef]
- Aswar, U.M.; Bhaskaran, S.; Mohan, V.; Bodhankar, S.L. Estrogenic activity of friedelin rich fraction (IND-HE) separated from Cissus quadrangularis and its effect on female sexual function. Pharmacogn. Res. 2010, 2, 138–145. [Google Scholar] [CrossRef]
- Emongor, V. Safflower (Carthamus tinctorius L.) the underutilized and neglected crop: A review. Asian J. Plant Sci. 2010, 9, 299–306. [Google Scholar] [CrossRef]
- Chavan, S.P.; Lokhande, V.H.; Nitnaware, K.M.; Nikam, T.D. Influence of growth regulators and elicitors on cell growth and α-tocopherol and pigment productions in cell cultures of Carthamus tinctorius L. Appl. Microbiol. Biotechnol. 2011, 89, 1701–1707. [Google Scholar] [CrossRef]
- Huh, J.S.; Kang, J.H.; Yoo, Y.J.; Kim, C.S.; Cho, K.S.; Choi, S.H. The effect of safflower seed fraction extract on periodontal ligament fibroblast and MC3T3-E1 cell in vitro. J. Korean Acad. Periodontol. 2001, 31, 833–846. [Google Scholar]
- Kim, S.T.; Jhon, G.J.; Lim, S.H.; Cho, K.S.; Kim, C.K.; Choi, S.H. The effect of safflower seed extract on the bone formation of calvarial bone model in Sprague Dawley rat. J. Korean Acad. Periodontol. 2000, 30, 835–850. [Google Scholar]
- You, K.T.; Choi, K.S.; Yun, G.Y.; Kim, E.C.; You, H.K.; Shin, H.S. Healing after implantation of bone substitutes and safflower seeds feeding in rat calvarial defects. J. Korean Acad. Periodontol. 2000, 30, 91–103. [Google Scholar]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Montgomery, K.S. Soy protein. J. Perinat. Educ. 2003, 12, 42–45. [Google Scholar]
- Perut, F.; Montufar, E.B.; Ciapetti, G.; Santin, M.; Salvage, J.; Traykova, T.; Planell, J.A.; Ginebra, M.P.; Baldini, N. Novel soybean/gelatine-based bioactive and injectable hydroxyapatite foam: Material properties and cell response. Acta Biomater. 2011, 7, 1780–1787. [Google Scholar] [CrossRef]
- Murkies, A.L.; Wilcox, G.; Davis, S.R. Clinical review 92: Phytoestrogens. J. Clin. Endocrinol. Metab. 1998, 83, 297–303. [Google Scholar] [CrossRef]
- Taku, K.; Melby, M.K.; Nishi, N.; Omori, T.; Kurzer, M.S. Soy isoflavones for osteoporosis: An evidence-based approach. Maturitas 2011, 70, 333–338. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Kuhnle, G.G.C.; Mulligan, A.A.; Touillaud, M.; Slimani, N.; Romieu, I.; Powell, N.; Tumino, R.; et al. Dietary intakes and food sources of phytoestrogens in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-hour dietary recall cohort. Eur. J. Clin. Nutr. 2012. [Google Scholar]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Santin, M.; Morris, C.; Standen, G.; Nicolais, L.; Ambrosio, L. A new class of bioactive and biodegradable soybean-based bone fillers. Biomacromolecules 2007, 8, 2706–2711. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Varoni, E.M.; Iriti, M.; Rimondini, L. Plant Products for Innovative Biomaterials in Dentistry. Coatings 2012, 2, 179-194. https://doi.org/10.3390/coatings2030179
Varoni EM, Iriti M, Rimondini L. Plant Products for Innovative Biomaterials in Dentistry. Coatings. 2012; 2(3):179-194. https://doi.org/10.3390/coatings2030179
Chicago/Turabian StyleVaroni, Elena M., Marcello Iriti, and Lia Rimondini. 2012. "Plant Products for Innovative Biomaterials in Dentistry" Coatings 2, no. 3: 179-194. https://doi.org/10.3390/coatings2030179
APA StyleVaroni, E. M., Iriti, M., & Rimondini, L. (2012). Plant Products for Innovative Biomaterials in Dentistry. Coatings, 2(3), 179-194. https://doi.org/10.3390/coatings2030179







