Paper-Based Microfluidic Platforms for Understanding the Role of Exosomes in the Pathogenesis of Major Blindness-Threatening Diseases
Abstract
:1. Introduction
2. Role of Exosomes in Ophthalmological Diseases
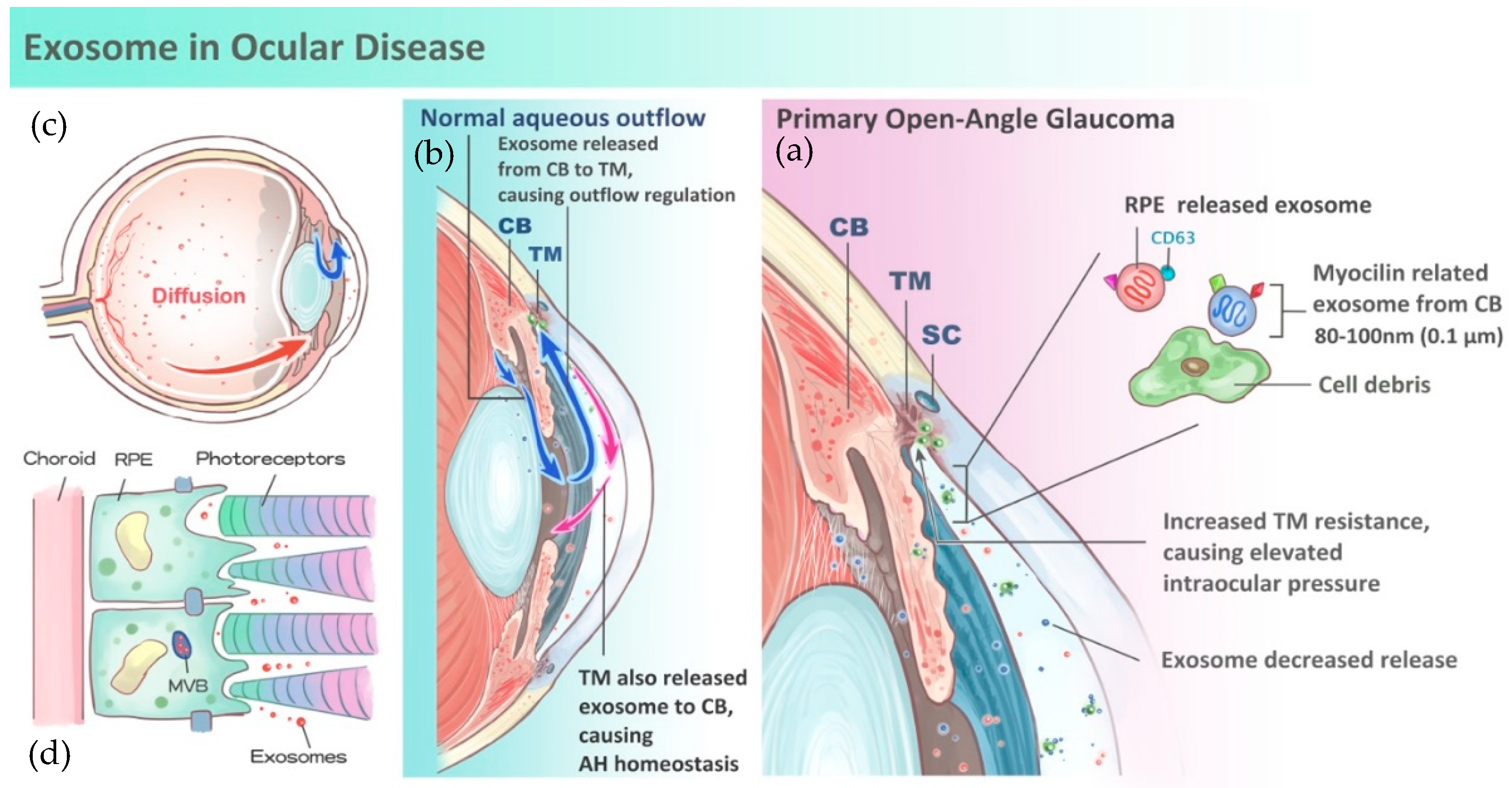
3. Role of Exosomes in Aqueous Humor Homeostasis and Glaucoma
4. Role of Exosomes in Age-Related Macular Degeneration
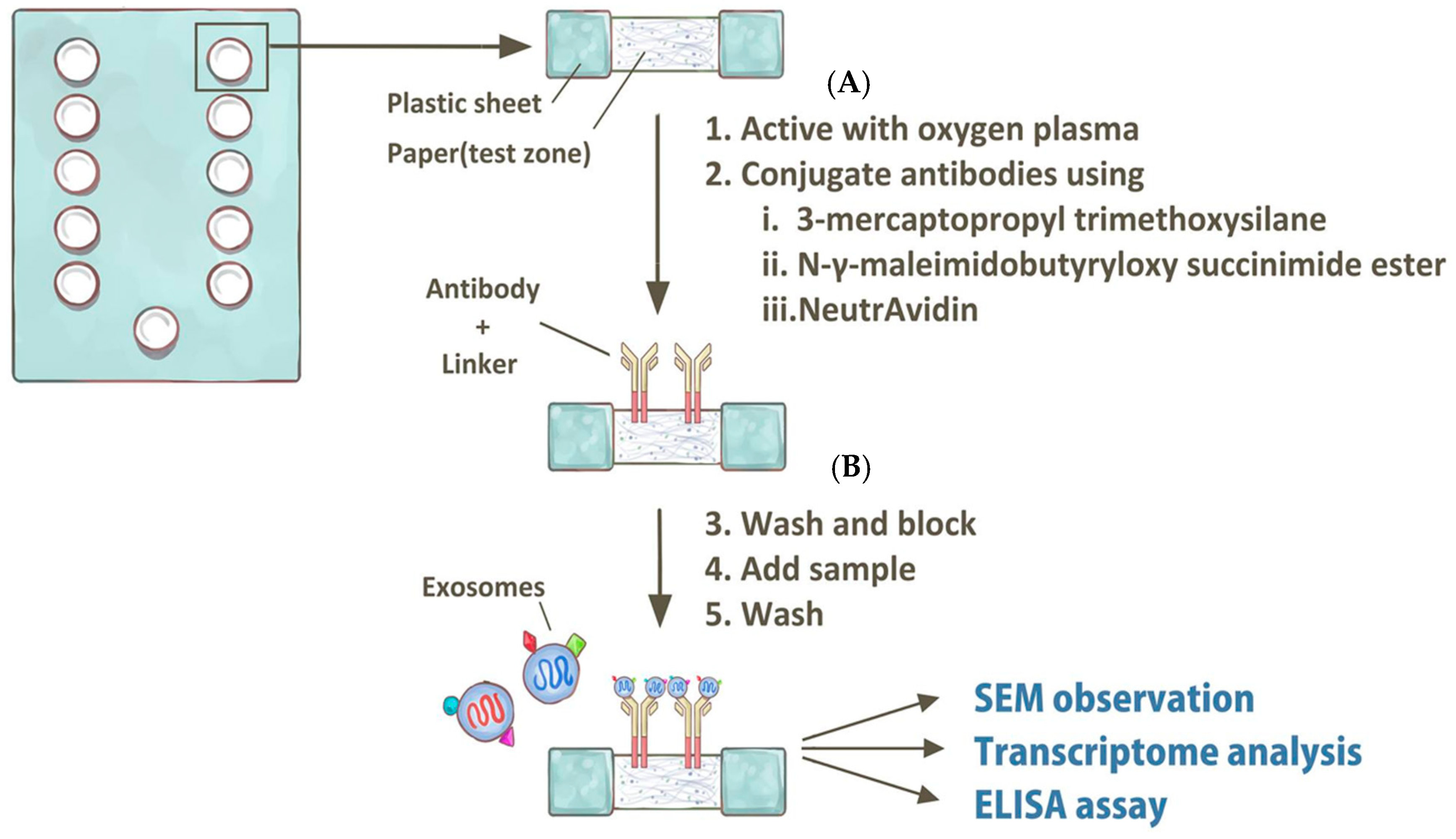
5. Perspectives—Current Research Limitations and Potential Breakthrough Using In Vitro Diagnostic Tools
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bin-Tao Pan, R.M.J. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [PubMed]
- Harding, V.C.; Heuser, J.E.; Stahl, P.D. Exosomes: Looking back three decades and into the future. J. Cell Biol. 2013, 200, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Azmi, S.A.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.; Gainche, L.; Sena-Esteves, M.; Curry, W.T.; Carter, R.S., Jr.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, B.H.; Smith, J.T.; Gifford, S.M.; Wang, C.; Brink, M.; Bruce, R.L.; Austin, R.H.; Stolovitzky, G.; Astier, Y. Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat. Nanotechnol. 2016, 11, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M. Exosomes and Microvesicles: Extracellular Frontiers of Intercellular Communication. In Encyclopedia of Surface and Colloid Science, 3rd ed.; CRC Press: New York, NY, USA, 2015; Volume 4, pp. 2632–2644. [Google Scholar]
- Jager, D.R.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and exosomes in the aged retinal pigment epithelium: Possible relevance to drusen formation and age-related macular degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.M.; Hoffman, E.A.; Gonzalez, P.; McKay, B.S.; Stamer, W.D. Extracellular trafficking of myocilin in human trabecular meshwork cells. J. Biol. Chem. 2005, 280, 28917–28926. [Google Scholar] [CrossRef] [PubMed]
- Resch, Z.T.; Hann, C.R.; Cook, K.A.; Fautsch, M.P. Aqueous humor rapidly stimulates myocilin secretion from human trabecular meshwork cells. Exp. Eye Res. 2010, 91, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.A.; Perkumas, K.M.; Highstrom, L.M.; Stamer, W.D. Regulation of myocilin-associated exosome release from human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Hoffman, E.A.; Luther, J.M.; Hachey, D.L.; Schey, K.L. Protein profile of exosomes from trabecular meshwork cells. J. Proteom. 2011, 74, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Perkumas, K.M.; Hoffman, E.A.; McKay, B.S.; Allingham, R.R.; Stamer, W.D. Myocilin-associated exosomes in human ocular samples. Exp. Eye Res. 2007, 84, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Savinova, O.V.; Reedy, M.V.; Martin, J.; Lun, Y.; Gan, L.; Smith, R.S.; Tomarev, S.I.; John, S.W.; Johnson, R.L. Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol. Cell. Biol. 2001, 21, 7707–7713. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wei, E.; Wang, X.; Zhang, X.; Morrison, J.C.; Parikh, M.; Lombardi, L.H.; Gattey, D.M.; Armour, R.L.; Edmunds, B. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014, 121, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Chiechi, A.; Couch, R.; Liotta, L.A.; Espina, V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp. Cell Res. 2013, 319, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Bird, A.C.; Bressler, N.M.; Bressler, S.B.; Chisholm, I.H.; Coscas, G.; Davis, M.D.; de Jong, P.T.; Klaver, C.C.; Klein, B.E.; Klein, R. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv. Ophthalmol. 1995, 39, 367–374. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Han, K.Y.; Tran, J.A.; Chang, J.-H.; Azar, D.T.; Zieske, J.D. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef] [PubMed]
- Han, K.Y.; Dugas-Ford, J.; Seiki, M.; Chang, J.H.; Azar, D.T. Evidence for the Involvement of MMP14 in MMP2 Processing and Recruitment in Exosomes of Corneal Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Chen, S.J.; Chen, K.H.; Hung, Y.C.; Tsai, H.Y.; Cheng, C.M. Monitoring VEGF levels with low-volume sampling in major vision-threatening diseases: Age-related macular degeneration and diabetic retinopathy. Lab Chip 2015, 15, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Hung, Y.C.; Hwang, D.K.; Lin, S.C.; Lin, K.H.; Wang, C.Y.; Choi, H.Y.; Wang, Y.P.; Cheng, C.M. Detection of aqueous VEGF concentrations before and after intravitreal injection of anti-VEGF antibody using low-volume sampling paper-based ELISA. Sci. Rep. 2016, 6, 34631. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, B.R.; Hsu, M.Y.; Cheng, C.M. Paper-based devices for isolation and characterization of extracellular vesicles. J. Vis. Exp. 2015, e52722. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, B.R.; Hsu, M.Y.; Cheng, C.M. Paper-based immunoaffinity devices for accessible isolation and characterization of extracellular vesicles. Microfluid. Nanofluid. 2014, 16, 849–856. [Google Scholar] [CrossRef]
- Green, J.V.; Sun, D.; Hafezi-Moghadam, A.; Lashkari, K.; Murthy, S.K. Microfluidic pillar array sandwich immunofluorescence assay for ocular diagnostics. Biomed. Microdevice 2011, 13, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Devadhasan, J.P.; Howse, R.; Kim, J.A. Chemically Patterned Microfluidic Paper-based Analytical Device (C-microPAD) for Point-of-Care Diagnostics. Sci. Rep. 2017, 7, 1188. [Google Scholar] [CrossRef] [PubMed]
- Kingeborn, M.; Dismuke, W.M.; Bowes Rickman, C.; Stamer, W.D. Roles of exosomes in the normal and diseased eye. Prog. Retin. Eye Res. 2017, 59, 158–177. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-Y.; Chiu, C.-C.; Wang, J.-Y.; Huang, C.-T.; Huang, Y.-F.; Liou, J.-C.; Chen, C.; Chen, H.-C.; Cheng, C.-M. Paper-Based Microfluidic Platforms for Understanding the Role of Exosomes in the Pathogenesis of Major Blindness-Threatening Diseases. Nanomaterials 2018, 8, 310. https://doi.org/10.3390/nano8050310
Hsu M-Y, Chiu C-C, Wang J-Y, Huang C-T, Huang Y-F, Liou J-C, Chen C, Chen H-C, Cheng C-M. Paper-Based Microfluidic Platforms for Understanding the Role of Exosomes in the Pathogenesis of Major Blindness-Threatening Diseases. Nanomaterials. 2018; 8(5):310. https://doi.org/10.3390/nano8050310
Chicago/Turabian StyleHsu, Min-Yen, Chun-Chih Chiu, Juan-Yuan Wang, Chin-Te Huang, Yu-Fang Huang, Jyh-Cheng Liou, Chihchen Chen, Hung-Chi Chen, and Chao-Min Cheng. 2018. "Paper-Based Microfluidic Platforms for Understanding the Role of Exosomes in the Pathogenesis of Major Blindness-Threatening Diseases" Nanomaterials 8, no. 5: 310. https://doi.org/10.3390/nano8050310







