The Influence of Modified Silica Nanomaterials on Adult Stem Cell Culture
Abstract
:1. Introduction
2. Results and Discussion
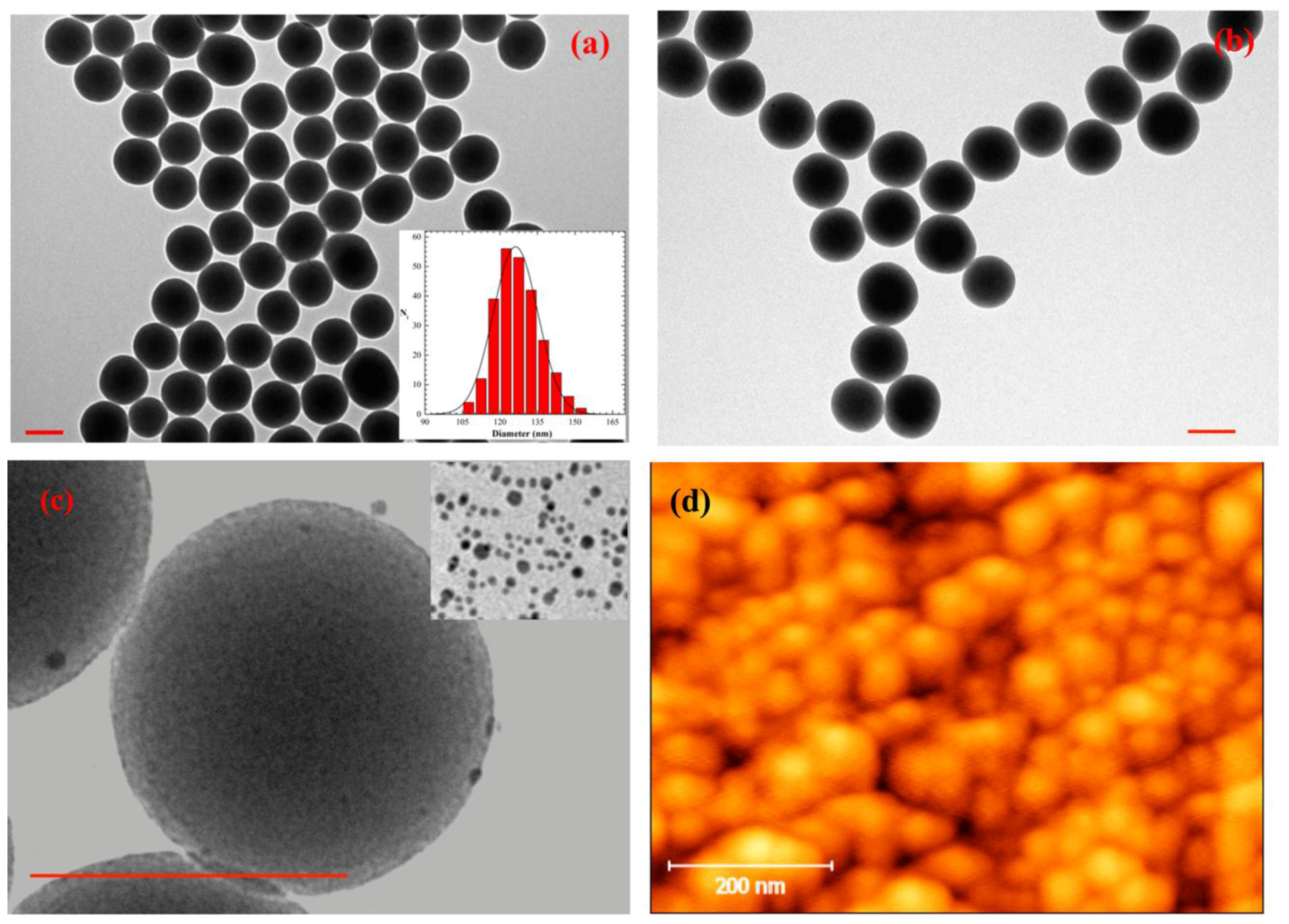
2.1. Morphological Characterization of the Substrates
2.2. Biological Characterization
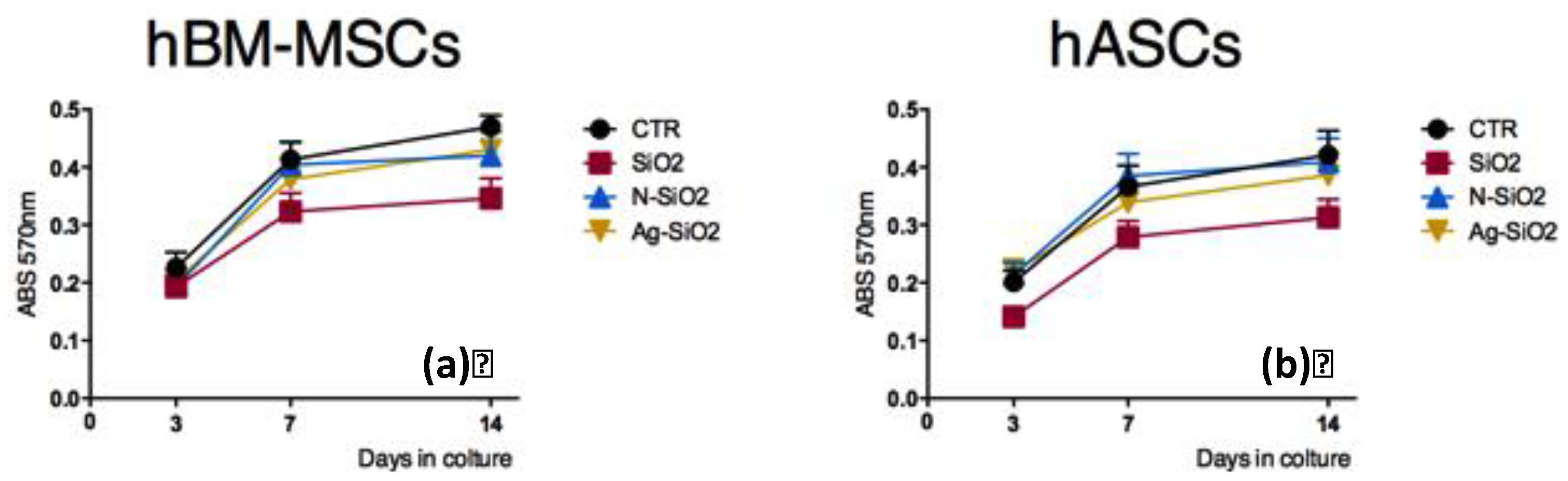
2.2.1. Stem Cell Viability on Surfaces of Differently Functionalized Silica Nanoparticles
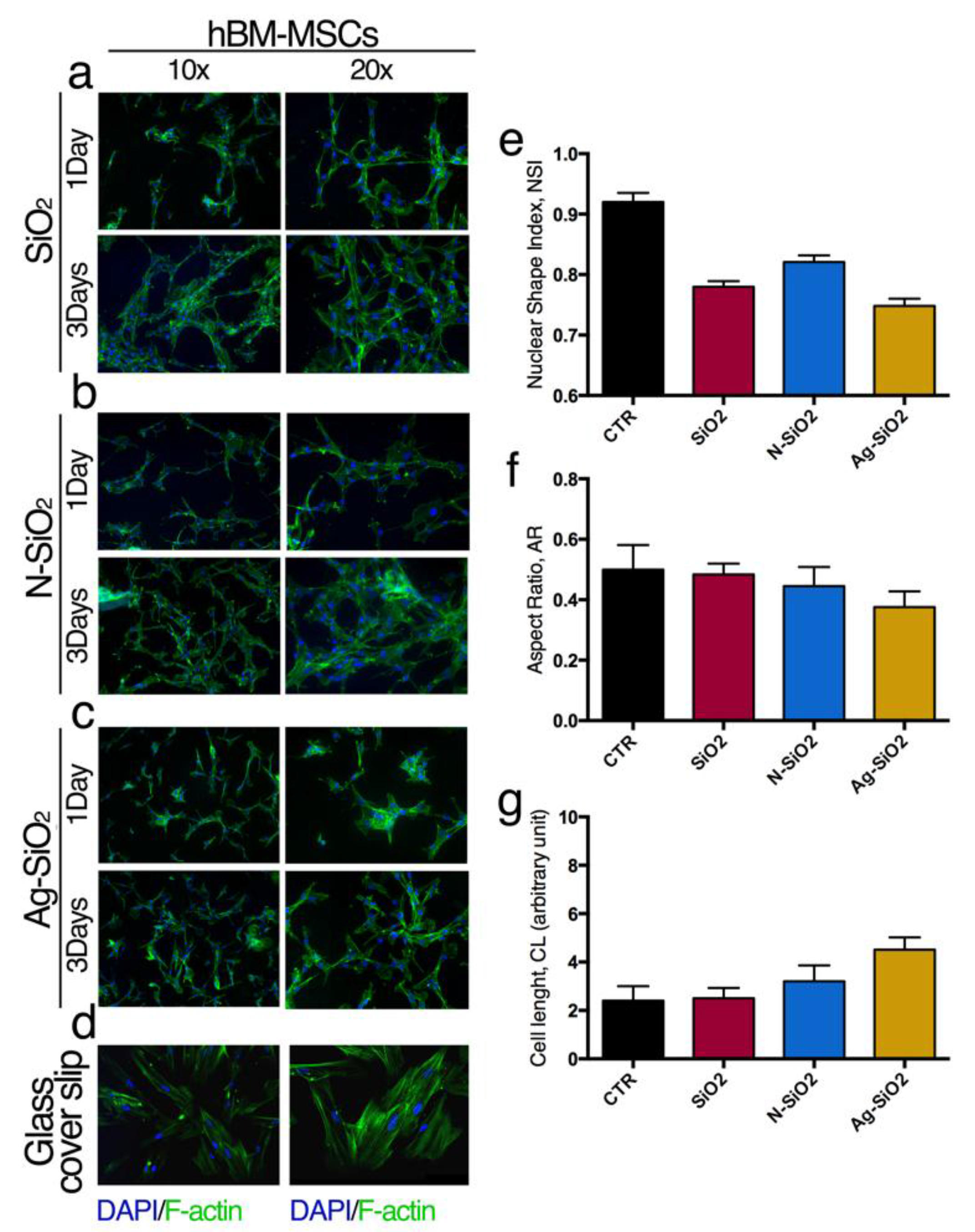
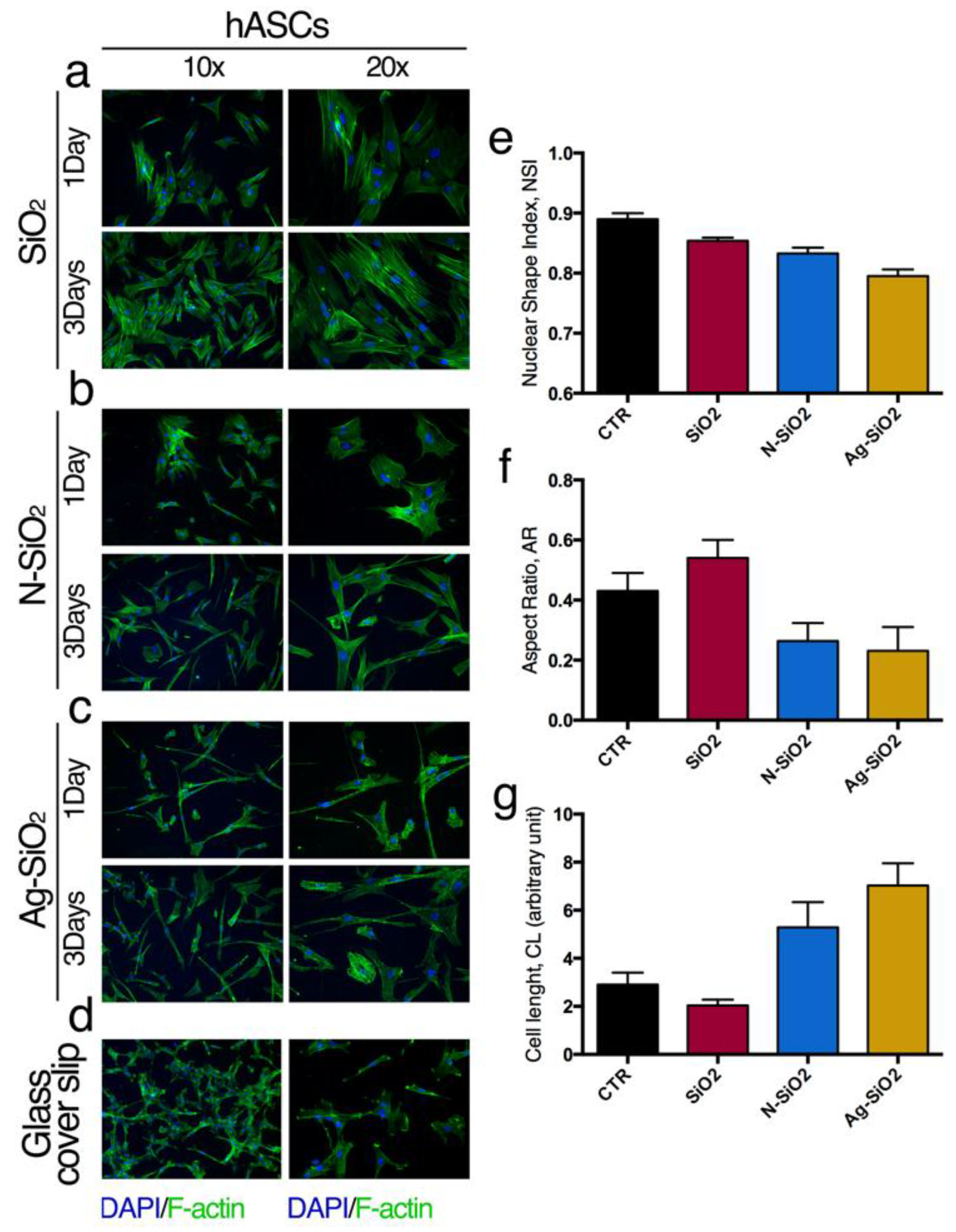
2.2.2. Interaction of Adult Stem Cells with Surfaces of Differently Functionalized Silica Nanoparticles
3. Experimental Section
3.1. Materials
3.2. Synthesis Procedures
3.2.1. Preparation of Silica Colloids
3.2.2. Preparation of Ag Nanoparticles
3.2.3. Preparation of the Ag/SiO2 Nanocomposite
3.2.4. Characterization of the Colloids
3.3. Biological Evaluation
3.3.1. Isolation and Culture of Human Bone Marrow-Mesenchymal Stem Cells
3.3.2. Isolation and Culture of Human Adipose-Derived Stem Cells from Lipoaspirate
3.3.3. Culture of Stem Cells on the Surfaces of SiO2, N-SiO2, and Ag-SiO2 Nanoparticles
3.3.4. Cell Viability Assay
3.3.5. Immunofluorescence
3.3.6. Cell and Nuclei Shape Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Seidi, A.; Sampathkumar, K.; Srivastava, A.; Ramakrishna, S.; Ramalingam, M. Gradient nanofiber scaffolds for tissue engineering. J. Nanosc. Nanotech. 2013, 13, 4647–4655. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, T.; He, N.; Wang, J.; Chen, W.; He, L.; Huang, C.; El-Hamshary, H.A.; Al-Deyab, S.S.; Ke, Q.; et al. Three-dimensional polycaprolactone scaffold via needleless electrospinning promotes cell proliferation and infiltration. Coll. Surf. B 2014, 121, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.M.Z.; Hasan, M.S.; Boyd, D.; Rudd, C.D.; Ahmed, I.; Thielemans, W. Effect of cellulose nanowhiskers on surface morphology, mechanical properties, and cell adhesion of melt-drawn polylactic acid fibers. Biomacromolecules 2014, 15, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Martínez-Carmona, M.; Sánchez-Salcedo, S.; Ruiz-González, M.L.; González-Calbet, J.M.; Vallet-Regí, M. A novel zwitterionic bioceramic with dual antibacterial capability. J. Mater. Chem. B 2014, 2, 5639–5651. [Google Scholar] [CrossRef]
- Halas, N.J. Nanoscience under Glass: The Versatile Chemistry of Silica Nanostructures. ACS Nano 2008, 2, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Liu, J.; Wang, L.Q.; Jiang, Y.; Lambert, T.N.; Fang, E. A new class of silica cross-linked micellar core-shell nanoparticles. J. Am. Chem. Soc. 2006, 128, 6447–6453. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, V.; Perioli, L.; Pagano, C.; Latterini, L.; Marmottini, F.; Ricci, M.; Rossi, C. MCM-41 for furosemide dissolution improvement. Microp. Mesop. Mater. 2012, 147, 343–349. [Google Scholar] [CrossRef]
- Latterini, L.; Tarpani, L. Hierarchical Assembly of Nanostructures to Decouple Fluorescence and Photothermal Effect. J. Phys. Chem. C 2011, 115, 21098–21104. [Google Scholar] [CrossRef]
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.L.; Chang, H.Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Latterini, L.; Amelia, M. Sensing Proteins with Luminescent Silica Nanoparticles. Langmuir 2009, 25, 4767–4773. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Khademi, C.; Gambhir, S.S. Intracellular aggregation of multimodal silica nanoparticles for ultrasound-guided stem cell implantation. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, V.; Donnadio, A.; Pietrella, D.; Latterini, L.; Proietti, F.A.; Marmottini, F.; Padeletti, G.; Kaciulis, S.; Giovagnoli, S.; Ricci, M. Chitosan films containing mesoporous SBA-15 supported silver nanoparticles for wound dressing. J. Mater. Chem. B 2014, 2, 6054–6063. [Google Scholar] [CrossRef]
- Martino, S.; D’Angelo, F.; Armentano, I.; Kenny, J.M.; Orlacchio, A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012, 30, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Martino, S.; Morena, F.; Barola, C.; Bicchi, I.; Emiliani, C. Proteomics and epigenetic mechanisms in stem cells. Curr. Proteomics 2014, 11, 193–209. [Google Scholar] [CrossRef]
- Martino, S.; D’Angelo, F.; Armentano, I.; Tiribuzi, R.; Pennacchi, M.; Dottori, M.S.; Mattioli, S.; Caraffa, A.; Cerulli, G.G.; Kenny, J.M.; et al. Hydrogenated amorphous carbon nanopatterned film designs drive human bone marrow mesenchymal stem cell cytoskeleton architecture. Tissue Eng. 2009, 827, 3139–3149. [Google Scholar] [CrossRef] [PubMed]
- Morena, F.; Argentati, C.; Calzoni, E.; Cordellini, M.; Emiliani, C.; D’Angelo, F.; Martino, S. Ex-Vivo tissues engineering modeling for reconstructive surgery using human adult adipose stem cells and polymeric nanostructured matrix. Nanomaterials 2016, 6. [Google Scholar] [CrossRef]
- D’Angelo, F.; Armentano, I.; Cacciotti, I.; Tiribuzi, R.; Quattrocelli, M.; Del Gaudio, C.; Fortunati, E.; Saino, E.; Caraffa, A.; Cerulli, G.G.; et al. Tuning multi/pluri-potent stem cell fate by electrospun poly(L-lactic acid)-calcium-deficient hydroxyapatite nanocomposite mats. Biomacromolecules 2012, 13, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, R.; Tarpani, L.; Santuari, A.; Giovagnoli, S.; Latterini, L. Silica nanoparticles assisted photodegradation of acridine orange in aqueous suspensions. Appl. Catal. B 2015, 168–169, 363–369. [Google Scholar] [CrossRef]
- Chen, G.S.; Chen, C.N.; Tseng, T.T.; Wei, M.H.; Hsieh, J.H.; Tseng, W.J. Synthesis, characterization, and antibacterial activity of silver-doped silica nanocomposite particles. J. Nanosci. Nanotechnol. 2011, 11, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Kim, K. Comparative study of dodecanethiol-derivatized silver nanoparticles prepared in one-phase and two-phase systems. Langmuir 1998, 14, 226–230. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarpani, L.; Morena, F.; Gambucci, M.; Zampini, G.; Massaro, G.; Argentati, C.; Emiliani, C.; Martino, S.; Latterini, L. The Influence of Modified Silica Nanomaterials on Adult Stem Cell Culture. Nanomaterials 2016, 6, 104. https://doi.org/10.3390/nano6060104
Tarpani L, Morena F, Gambucci M, Zampini G, Massaro G, Argentati C, Emiliani C, Martino S, Latterini L. The Influence of Modified Silica Nanomaterials on Adult Stem Cell Culture. Nanomaterials. 2016; 6(6):104. https://doi.org/10.3390/nano6060104
Chicago/Turabian StyleTarpani, Luigi, Francesco Morena, Marta Gambucci, Giulia Zampini, Giuseppina Massaro, Chiara Argentati, Carla Emiliani, Sabata Martino, and Loredana Latterini. 2016. "The Influence of Modified Silica Nanomaterials on Adult Stem Cell Culture" Nanomaterials 6, no. 6: 104. https://doi.org/10.3390/nano6060104