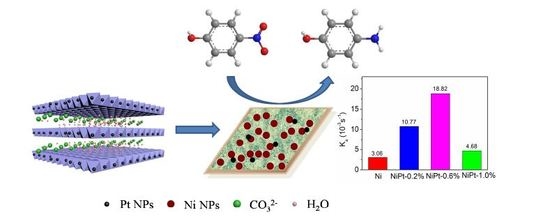
Enhanced Activity of Supported Ni Catalysts Promoted by Pt for Rapid Reduction of Aromatic Nitro Compounds
Abstract
:1. Introduction
2. Results and Discussions
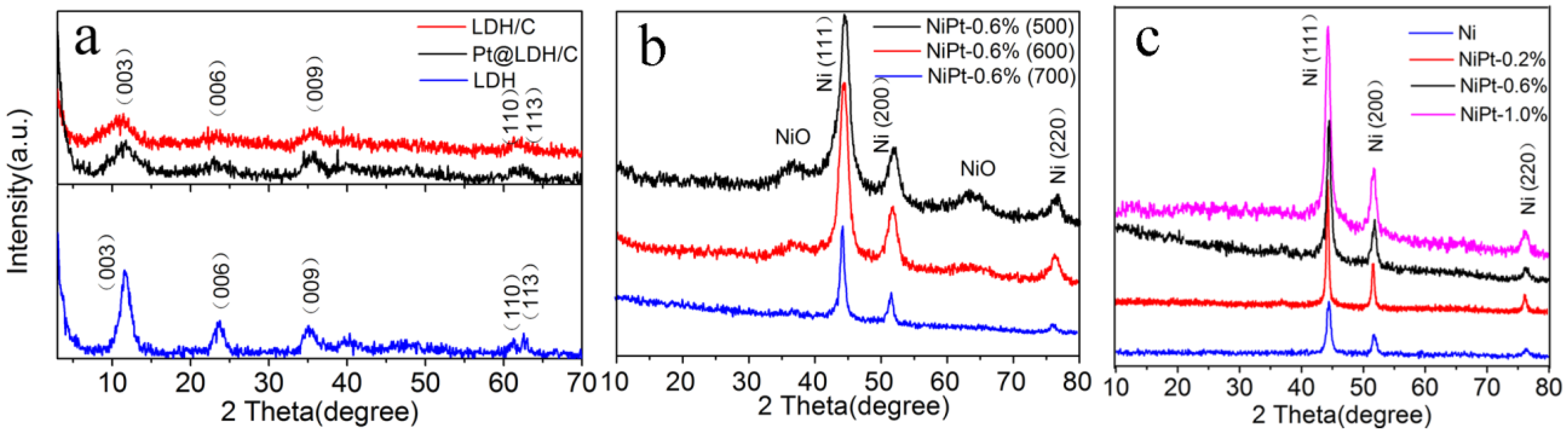
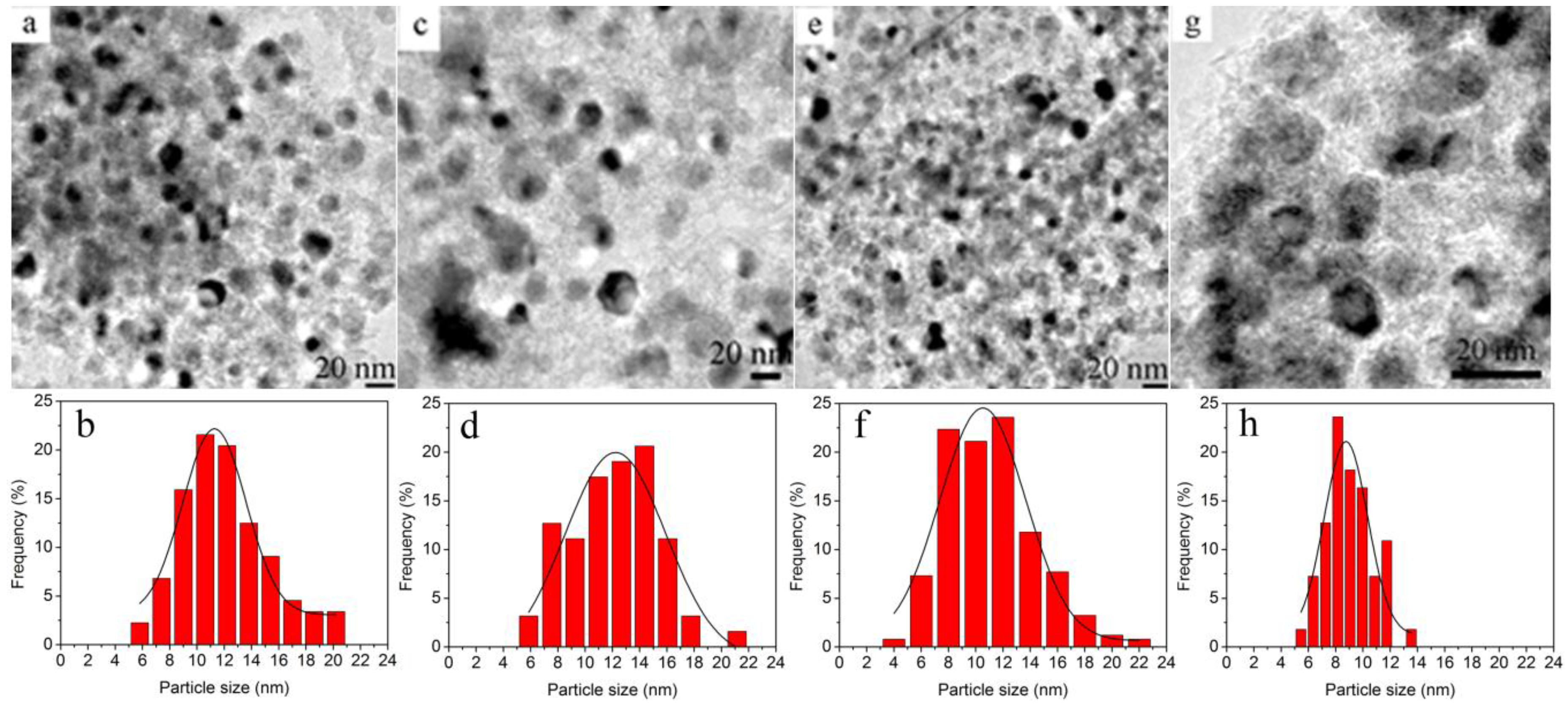
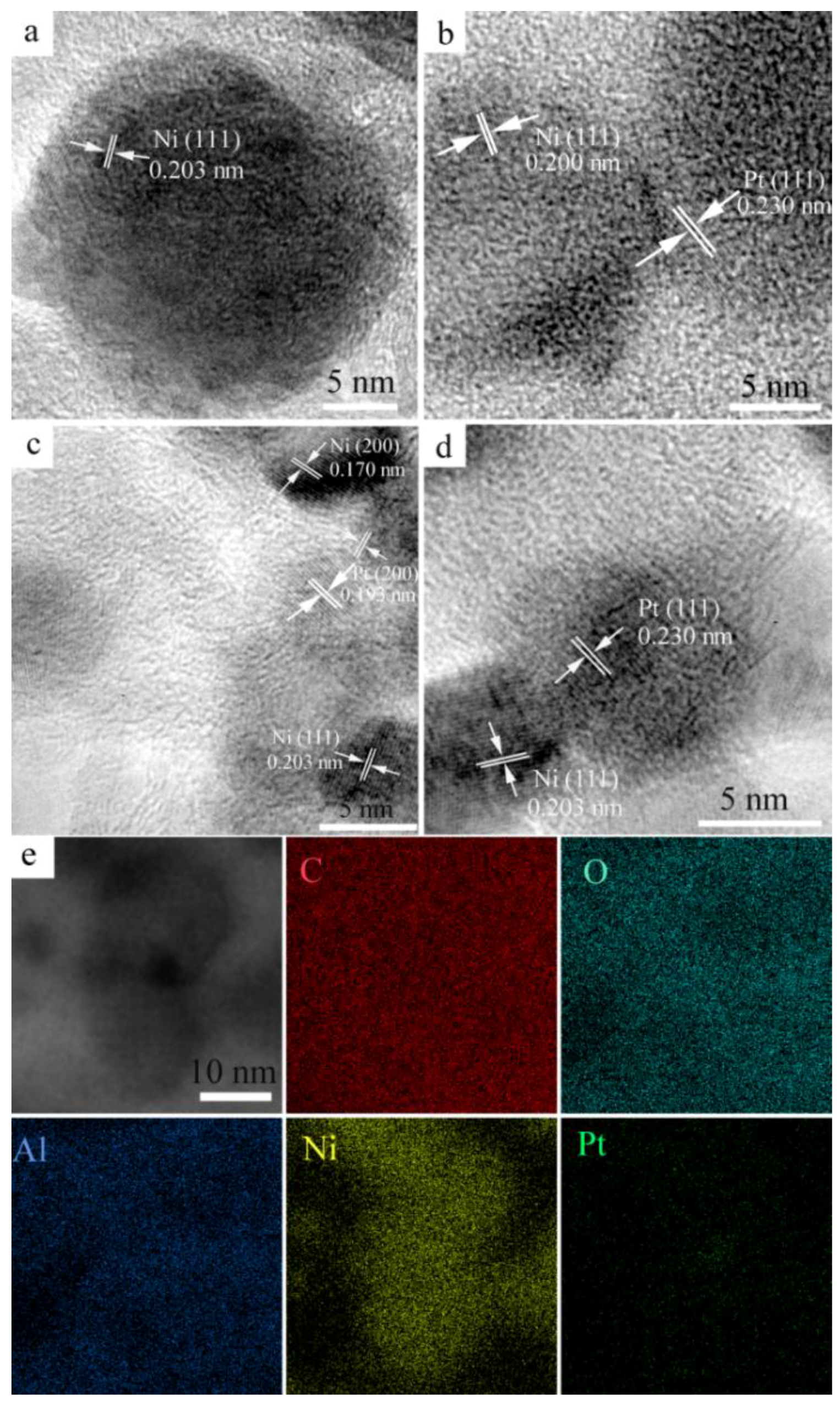
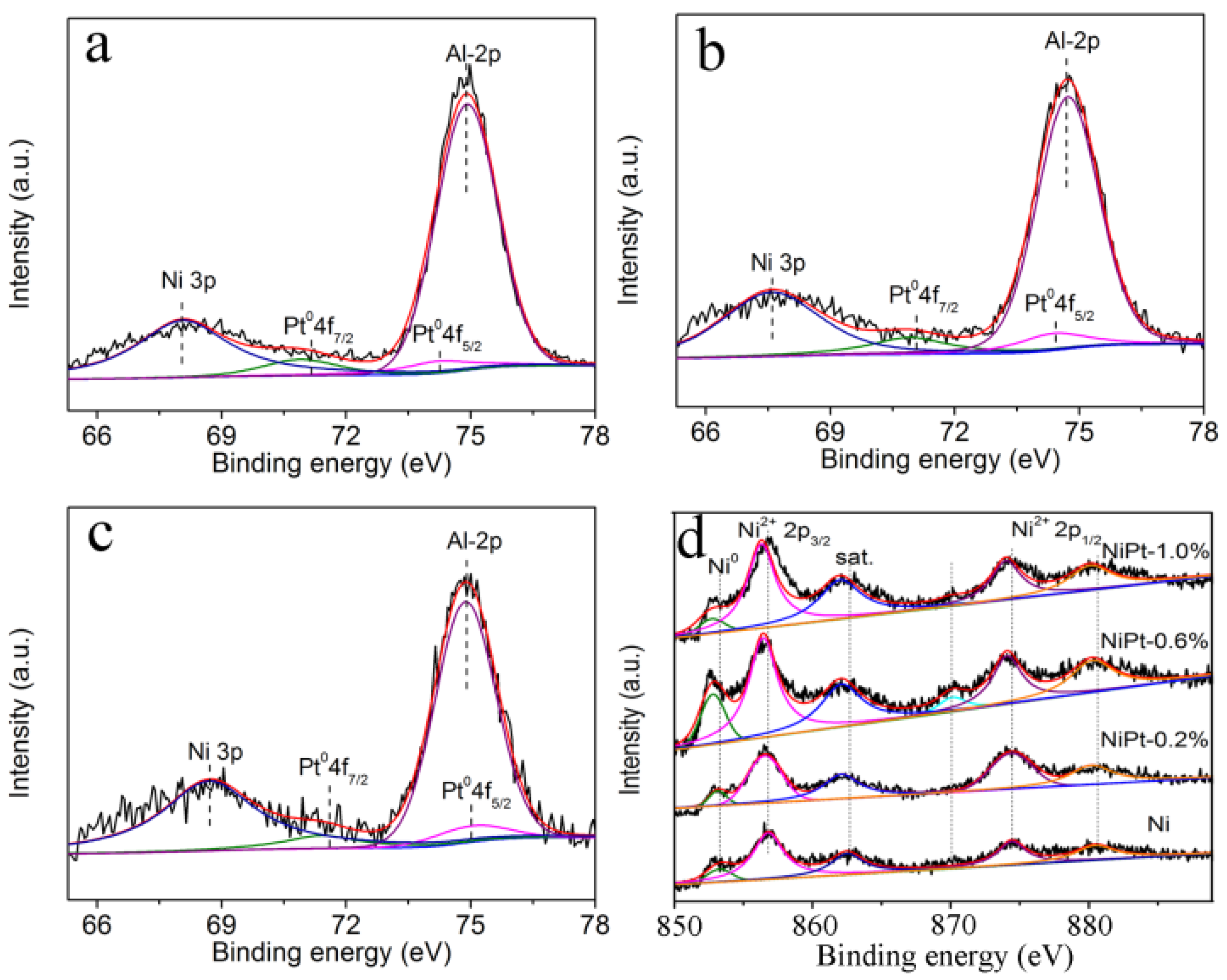
2.1. Characterization of Materials
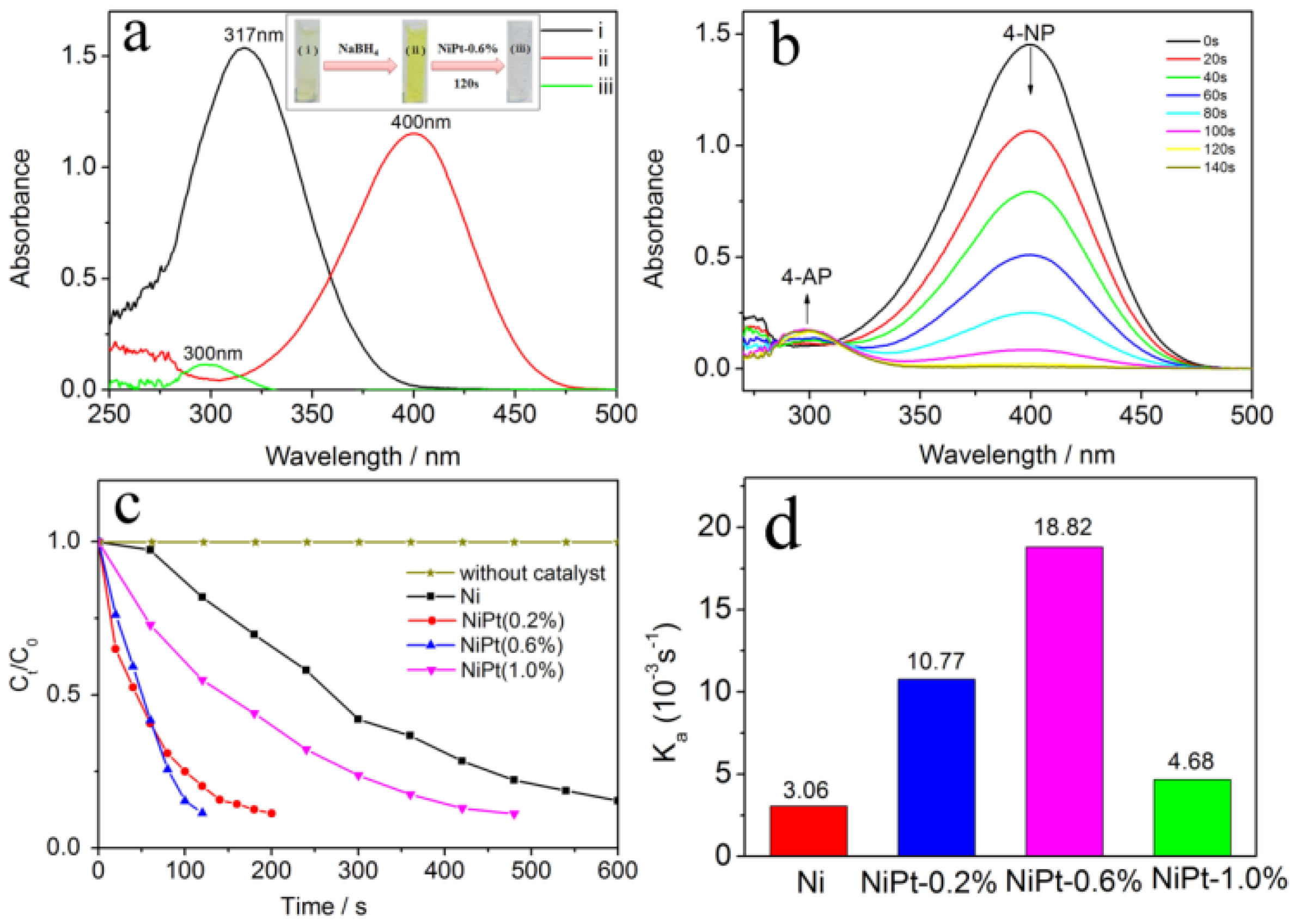
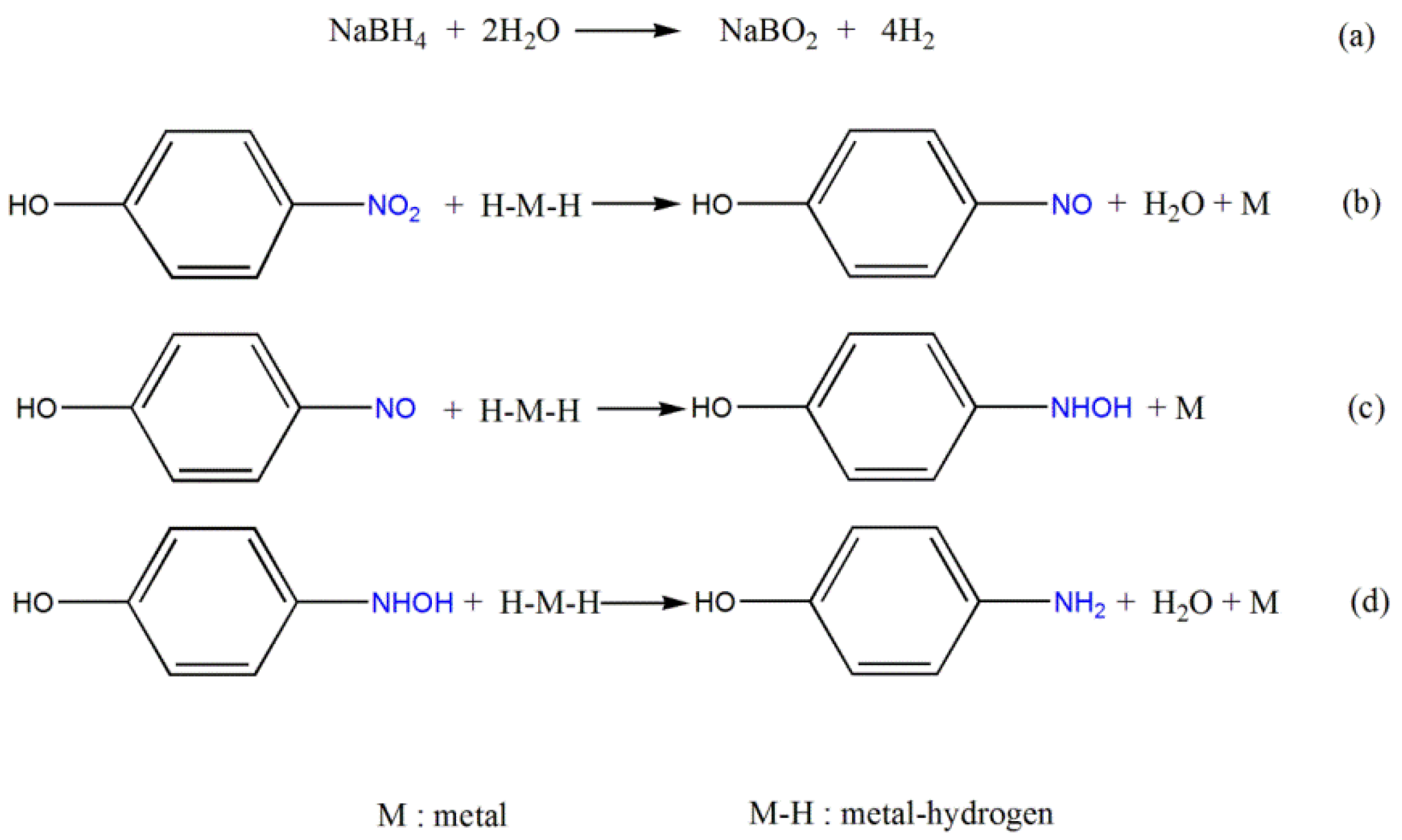
2.2. Evaluation of the Catalytic Activity
3. Experimental Section
3.1. Materials
3.2. Synthesis of NiAl-LDH Precursor
3.3. Synthesis of LDH/Carbon Composites and Supported NiPt Catalysts
3.4. Characterizations
3.5. Catalytic Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, J.; Fu, Y.; He, G.; Sun, X.; Wang, X. Catalytic hydrogenation of nitrophenols and nitrotoluenes over a palladium/graphene nanocomposite. Catal. Sci. Technol. 2014, 4, 1742–1748. [Google Scholar] [CrossRef]
- Xu, D.; Diao, P.; Jin, T.; Wu, Q.; Liu, X.; Guo, X.; Gong, H.; Li, F.; Xiang, M.; Ronghai, Y. Iridium oxide nanoparticles and iridium/iridium oxide nanocomposites: Photochemical fabrication and application in catalytic reduction of 4-nitrophenol. ACS Appl. Mater. Interfaces 2015, 7, 16738–16749. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Tian, K.; Jiang, H. One-pot synthesis of Ni–NiFe2O4/carbon nanofiber composites from biomass for selective hydrogenation of aromatic nitro compounds. Green Chem. 2015, 17, 821–826. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, S.; Sun, Y.; Gai, S.; He, F.; Dai, Y.; Li, L.; Yang, P. Controllable synthesis of Ni/SiO2 hollow spheres and their excellent catalytic performance in 4-nitrophenol reduction. Dalton Trans. 2014, 43, 16911–16918. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Pramod, C.; Suresh, M.; Reddy, K.H.P.; Raju, B.D.; Rao, K.R. Advantage of Ni/SBA-15 catalyst over Ni/MgO catalyst in terms of catalyst stability due to release of water during nitrobenzene hydrogenation to aniline. Catal. Commun. 2012, 18, 89–92. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, J.; Jiang, D.; Wei, X.; Chen, M. Modifiers-assisted formation of nickel nanoparticles and their catalytic application to p-nitrophenol reduction. CrystEngComm 2013, 15, 560–569. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, L.; Ge, G. A green approach for efficient p-nitrophenol hydrogenation catalyzed by a Pd-based nanocatalyst. Catal. Commun. 2015, 66, 95–99. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Joshi, C.; Manchanda, M.; Boukherroub, R.; Jain, S.L. Nickel decorated on phosphorous-doped carbon nitride as an efficient photocatalyst for reduction of nitrobenzenes. Nanomaterials 2016, 6. [Google Scholar] [CrossRef]
- Deka, P.; Deka, R.C.; Bharali, P. In situ generated copper nanoparticle catalyzed reduction of 4-nitrophenol. New J. Chem. 2014, 38, 1789–1793. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, X.; Zheng, Z.; Cheng, X.; Peng, Y. Template-free method to prepare polymer nanocapsules embedded with noble metal nanoparticles. Chem. Commun. 2007, 36, 3720–3722. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.-B.; Wang, Z.-L.; Wang, L.-M.; Xing, W.; Liu, X. One-step and rapid synthesis of “clean” and monodisperse dendritic Pt nanoparticles and their high performance toward methanol oxidation and p-nitrophenol reduction. Nanoscale 2012, 4, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Baruah, B.; Gabriel, G.J.; Akbashev, M.J.; Booher, M.E. Facile synthesis of silver nanoparticles stabilized by cationic polynorbornenes and their catalytic activity in 4-nitrophenol reduction. Langmuir 2013, 29, 4225–4234. [Google Scholar] [CrossRef] [PubMed]
- Gangula, A.; Podila, R.; Karanam, L.; Janardhana, C.; Rao, A.M. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir 2011, 27, 15268–15274. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, J.; Di, Q.; Zhang, M. Size-controlled synthesis of a supported Ni nanoparticle catalyst for selective hydrogenation of p-nitrophenol to p-aminophenol. Catal. Commun. 2012, 18, 55–59. [Google Scholar] [CrossRef]
- Zhang, S.; Gai, S.; He, F.; Dai, Y.; Gao, P.; Li, L.; Chen, Y.; Yang, P. Uniform Ni/SiO2@Au magnetic hollow microspheres: Rational design and excellent catalytic performance in 4-nitrophenol reduction. Nanoscale 2014, 6, 7025–7032. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yamashita, H.; Chen, X.; Yamanaka, J.; Kotobuki, M.; Suzuki, H.; Uchida, H. Nano-sized Ni particles on hollow alumina ball: Catalysts for hydrogen production. Appl. Catal. B Environ. 2007, 71, 237–245. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The golden age of transfer hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Cheng, H.; He, L.; Yu, Y.; Zhao, F. High performance of Ir-promoted Ni/TiO2 catalyst toward the selective hydrogenation of cinnamaldehyde. J. Catal. 2013, 303, 110–116. [Google Scholar] [CrossRef]
- Wang, S.; Lin, W.; Zhu, Y.; Xie, Y.; McCormick, J.R.; Huang, W.; Chen, J.G. Pd-based bimetallic catalysts prepared by replacement reactions. Catal. Lett. 2007, 114, 169–173. [Google Scholar] [CrossRef]
- Zhao, Z.-F.; Wu, Z.-J.; Zhou, L.-X.; Zhang, M.-H.; Li, W.; Tao, K.-Y. Synthesis of a nano-nickel catalyst modified by ruthenium for hydrogenation and hydrodechlorination. Catal. Commun. 2008, 9, 2191–2194. [Google Scholar] [CrossRef]
- Nishikawa, J.; Miyazawa, T.; Nakamura, K.; Asadullah, M.; Kunimori, K.; Tomishige, K. Promoting effect of Pt addition to Ni/CeO2/Al2O3 catalyst for steam gasification of biomass. Catal. Commun. 2008, 9, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Xiang, X.; Bai, L.; Li, F. Formation and catalytic performance of supported Ni nanoparticles via self-reduction of hybrid NiAl-LDH/C composites. AIChE J. 2010, 56, 2934–2945. [Google Scholar] [CrossRef]
- Wang, Y.; He, W.; Wang, L.; Yang, J.; Xiang, X.; Zhang, B.; Li, F. Highly active supported Pt nanocatalysts synthesized by alcohol reduction towards hydrogenation of cinnamaldehyde: Synergy of metal valence and hydroxyl groups. Chem. Asian J. 2015, 10, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; He, W.; Xie, L.; Li, F. A mild solution chemistry method to synthesize hydrotalcite-supported platinum nanocrystals for selective hydrogenation of cinnamaldehyde in neat water. Catal. Sci. Technol. 2013, 3, 2819–2827. [Google Scholar] [CrossRef]
- Xiang, X.; Hima, H.I.; Wang, H.; Li, F. Facile Synthesis and Catalytic Properties of nickel-based mixed-metal oxides with mesopore networks from a novel hybrid composite precursor. Chem. Mater. 2007, 20, 1173–1182. [Google Scholar] [CrossRef]
- Xiang, X.; Li, F.; Huang, Z. Recent advances in layered double hydroxide-based materials as versatile photocatalysts. Rev. Adv. Sci. Eng. 2014, 3, 158–171. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.K.; Branco, P.S.; Nogueira, I.D.; Velhinho, A.; Shrikhande, J.J.; Indulkar, U.U.; Jayaram, R.V.; Ghumman, C.A.A.; Bundaleski, N. Regio-and chemoselective reduction of nitroarenes and carbonyl compounds over recyclable magnetic ferrite-nickel nanoparticles (Fe3O4-Ni) by using glycerol as a hydrogen source. Chem. Eur. J. 2012, 18, 12628–12632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Zhao, Y.; Zhang, B.; Yunyin, N.; Xiang, X.; Chen, R. Fabricating roughened surfaces on halloysite nanotubes via alkali etching for deposition of high-efficiency Pt nanocatalysts. CrystEngComm 2015, 17, 3110–3116. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef] [PubMed]
- Stassi, J.P.; Zgolicz, P.D.; de Miguel, S.R.; Scelza, O.A. Formation of different promoted metallic phases in PtFe and PtSn catalysts supported on carbonaceous materials used for selective hydrogenation. J. Catal. 2013, 306, 11–29. [Google Scholar] [CrossRef]
- Bera, P.; Priolkar, K.; Gayen, A.; Sarode, P.; Hegde, M.; Emura, S.; Kumashiro, R.; Jayaram, V.; Subbanna, G. Ionic dispersion of Pt over CeO2 by the combustion method: Structural investigation by XRD, TEM, XPS, and EXAFS. Chem. Mater. 2003, 15, 2049–2060. [Google Scholar] [CrossRef]
- Zhou, X.-W.; Zhang, R.-H.; Zhou, Z.-Y.; Sun, S.-G. Preparation of PtNi hollow nanospheres for the electrocatalytic oxidation of methanol. J. Power Sources 2011, 196, 5844–5848. [Google Scholar] [CrossRef]
- Pawelec, B.; Damyanova, S.; Arishtirova, K.; Fierro, J.; Petrov, L. Structural and surface features of PtNi catalysts for reforming of methane with CO2. Appl. Catal. A 2007, 323, 188–201. [Google Scholar] [CrossRef]
- Haber, J.A.; Cai, Y.; Jung, S.; Xiang, C.; Mitrovic, S.; Jin, J.; Bell, A.T.; Gregoire, J.M. Discovering Ce-rich oxygen evolution catalysts, from high throughput screening to water electrolysis. Energy Environ. Sci. 2014, 7, 682–688. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Panigrahy, B.; Bahadur, D. Facile synthesis of reduced graphene oxide/Pt–Ni nanocatalysts: Their magnetic and catalytic properties. RSC Adv. 2014, 4, 48563–48571. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Improving the hydrogenation function of Pd/γ-Al2O3 catalyst by Rh/γ-Al2O3 addition in CO2 methanation at low temperature. ACS Catal. 2013, 3, 2799–2812. [Google Scholar] [CrossRef]
- Dhar, J.; Patil, S. Self-assembly and catalytic activity of metal nanoparticles immobilized in polymer membrane prepared via layer-by-layer approach. ACS Appl. Mater. Interfaces 2012, 4, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Mandal, T.K. Synthesis and catalytic application of nanostructured silver dendrites. J. Phys. Chem. C 2007, 111, 16750–16760. [Google Scholar] [CrossRef]
- Xiao, Q.; Sarina, S.; Waclawik, E.R.; Jia, J.; Chang, J.; Riches, J.D.; Wu, H.; Zheng, Z.; Zhu, H. Alloying gold with copper makes for a highly selective visible-light photocatalyst for the reduction of nitroaromatics to anilines. ACS Catal. 2016, 6, 1744–1753. [Google Scholar] [CrossRef]
- Dandapat, A.; Jana, D.; De, G. Synthesis of thick mesoporous γ-alumina films, loading of Pt nanoparticles, and use of the composite film as a reusable catalyst. ACS Appl. Mater. Interfaces 2009, 1, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Na, T.; Liu, J.; Wenjie, S. Tuning the shape of ceria nanomaterials for catalytic applications. Chin. J. Catal. 2013, 34, 838–850. [Google Scholar]
- Ghosh, S.K.; Mandal, M.; Kundu, S.; Nath, S.; Pal, T. Bimetallic Pt–Ni nanoparticles can catalyze reduction of aromatic nitro compounds by sodium borohydride in aqueous solution. Appl. Catal. A 2004, 268, 61–66. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Zou, P.; Zhang, P.; Zhang, M.; Mu, J.; Guo, Z.; Li, X.; Wang, C.; Liu, Y. In situ assembly of well-dispersed gold nanoparticles on electrospun silica nanotubes for catalytic reduction of 4-nitrophenol. Chem. Commun. 2011, 47, 3906–3908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Chaker, M.; Rosei, F.; Ma, D. Gold nanoparticle decorated ceria nanotubes with significantly high catalytic activity for the reduction of nitrophenol and mechanism study. Appl. Catal. B 2013, 132, 107–115. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Bahn, S.; Hansen, L.B.; Bollinger, M.; Bengaard, H.; Hammer, B.; Sljivancanin, Z.; Mavrikakis, M. Universality in heterogeneous catalysis. J. Catal. 2002, 209, 275–278. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K.; Mavrikakis, M. Electronic structure and catalysis on metal surfaces. Annu. Rev. Phys. Chem. 2002, 53, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.P.; Lewera, A.; Larsen, R.; Masel, R.I.; Bagus, P.S.; Wieckowski, A. Size effects in electronic and catalytic properties of unsupported palladium nanoparticles in electrooxidation of formic acid. J. Phys. Chem. B 2006, 110, 13393–13398. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Chen, S.; Ding, W.; Xie, X.; Zhang, Y.; Wei, Z. Pt/C trapped in activated graphitic carbon layers as a highly durable electrocatalyst for the oxygen reduction reaction. Chem. Commun. 2014, 50, 15431–15434. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, D.; Liao, S.; Song, H.; Li, Y.; Fu, Z.; Su, Y. High-performance Pd–Au bimetallic catalyst with mesoporous silica nanoparticles as support and its catalysis of cinnamaldehyde hydrogenation. J. Catal. 2012, 291, 36–43. [Google Scholar] [CrossRef]
- Joseph, T.; Kumar, K.V.; Ramaswamy, A.; Halligudi, S. Au–Pt nanoparticles in amine functionalized MCM-41: Catalytic evaluation in hydrogenation reactions. Catal. Commun. 2007, 8, 629–634. [Google Scholar] [CrossRef]

| Catalyst | Pt wt % 1 | Ni wt % | D (nm) 2 | Specific Surface Area (m2 g−1) 3 |
|---|---|---|---|---|
| Ni | 0 | 35.85 | 12.1 | 244.6 |
| NiPt-0.2% | 0.167 | 38.75 | 12.1 | 271.8 |
| NiPt-0.6% | 0.347 | 36.98 | 11.0 | 266.0 |
| NiPt-1.0% | 0.537 | 39.55 | 9.0 | 267.4 |
| Catalysts | 2p3/2 (eV) | 2p1/2 (eV) | 4f7/2 (eV) | 4f5/2 (eV) | Ni3p | Al2p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ni0 | Ni2+ | NiSat. | Ni0 | Ni2+ | NiSat. | Pt0 | ||||
| Ni | 853.3 | 856.8 | 862.5 | 870.9 | 874.4 | 880.5 | - | - | - | 74.8 |
| NiPt-0.2% | 853.2 | 856.6 | 862.1 | 870.1 | 874.4 | 880.2 | 70.9 | 74.2 | 68.1 | 74.9 |
| NiPt-0.6% | 852.8 | 856.4 | 862.1 | 870.1 | 874.1 | 880.3 | 70.9 | 74.4 | 67.7 | 74.7 |
| NiPt-1.0% | 852.7 | 856.3 | 862.0 | 870.0 | 874.0 | 880.1 | 71.4 | 75.0 | 68.4 | 74.9 |
| Catalysts | Reaction Conditions 1 | Ka 2/10−3 s−1 | References |
|---|---|---|---|
| NiPt-0.6% (Ni: 36.98 wt % Pt: 0.347 wt %) | 15.5 mg, 2 mM, 25 °C, 0.25 M | 18.82 | This work |
| Pd0.05/G | 4 mg, 0.3 mM, 25 °C, 0.1 M | 36.5 | [1] |
| Ir/IrOx | -, 20 mM, 25 °C, 0.2 M | 2.57 | [2] |
| 230 nm Ni/SiO2 MHMs (Ni: 14.6 wt %) | 3 mg, 5 mM, 25 °C, 0.2 M | 4.5 | [4] |
| Ni NPs | 3 mg, 0.1mM, 20 °C, 0.2 M | 2.7 | [6] |
| Pd-Fe3O4 (1.2 wt %) | 10 mg, 21.56 mM, 25 °C, 0.1 MPa | - | [7] |
| DPNs | -, 2 mM, 25 °C, 0.3 M | 0.75 | [11] |
| Ni/TiO2 | 400 mg, -, 100 °C, 1.5 MPa | - | [14] |
| Ni/SiO2@Au MHMs | 4 mg, 5 mM, 25 °C, 0.2 M | 10 | [15] |
| RGO/PtNi (25:75) | 3 mg, 5 mM, 25 °C, 1.5 M | 1.12 | [35] |
| TAC-Ag-1.0 | 0.004 mg, 0.103 mM, 25 °C, 0.3 M | 5.19 | [38] |
| Au-Cu alloy NP | 50 mg, -, 60 °C, - | - | [39] |
| Pt/γ-Al2O3 (2.7 wt %) | 0.5 mg, 1 mM, 22 °C, 0.1M | 0.53 | [40] |
| Ni-Pt (96:4) | 0.004 mg, 0.085 mM, 25 °C, 0.012 M | 1.93 | [42] |
| AuNPs/SNTs | 8 mg, 0.12 mM, 25 °C, 0.005 M | 10.64 | [43] |
| AuNP/CeO2 (Au: 0.031 mg) | 10 mg, 0.12 mM, 25 °C, 0.005 M | 2.25 | [44] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, H.; Pan, K.; Zhang, L.; Zhang, B.; Xiang, X. Enhanced Activity of Supported Ni Catalysts Promoted by Pt for Rapid Reduction of Aromatic Nitro Compounds. Nanomaterials 2016, 6, 103. https://doi.org/10.3390/nano6060103
Shang H, Pan K, Zhang L, Zhang B, Xiang X. Enhanced Activity of Supported Ni Catalysts Promoted by Pt for Rapid Reduction of Aromatic Nitro Compounds. Nanomaterials. 2016; 6(6):103. https://doi.org/10.3390/nano6060103
Chicago/Turabian StyleShang, Huishan, Kecheng Pan, Lu Zhang, Bing Zhang, and Xu Xiang. 2016. "Enhanced Activity of Supported Ni Catalysts Promoted by Pt for Rapid Reduction of Aromatic Nitro Compounds" Nanomaterials 6, no. 6: 103. https://doi.org/10.3390/nano6060103