Temperature-Dependent Magnetic Response of Antiferromagnetic Doping in Cobalt Ferrite Nanostructures
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Structure and Phase Analysis
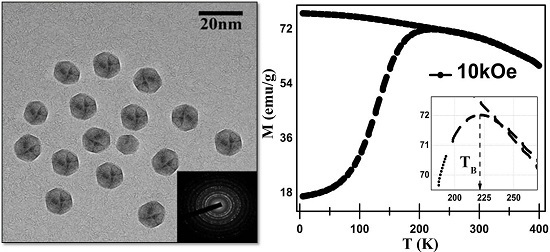
3.2. TEM Analysis
3.3. FTIR Spectroscopy
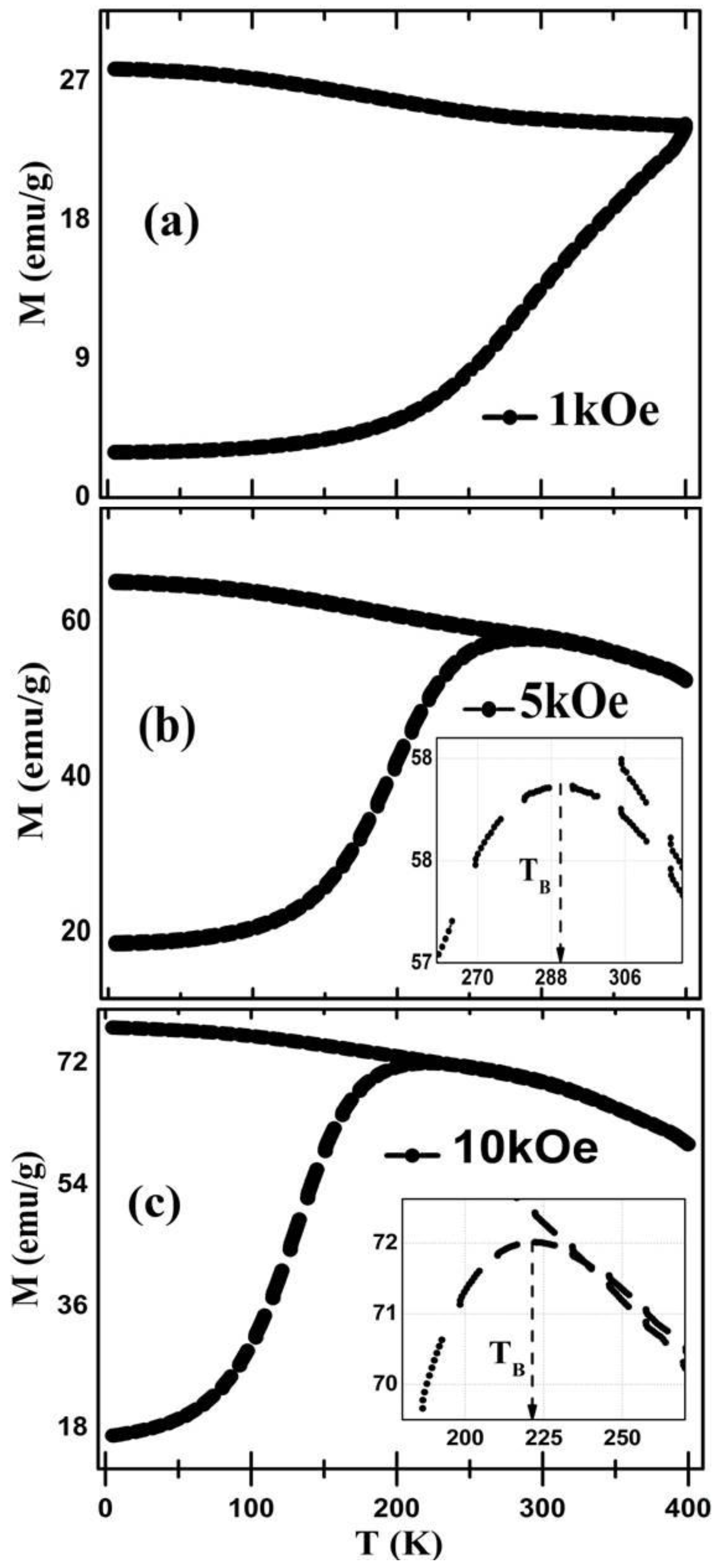
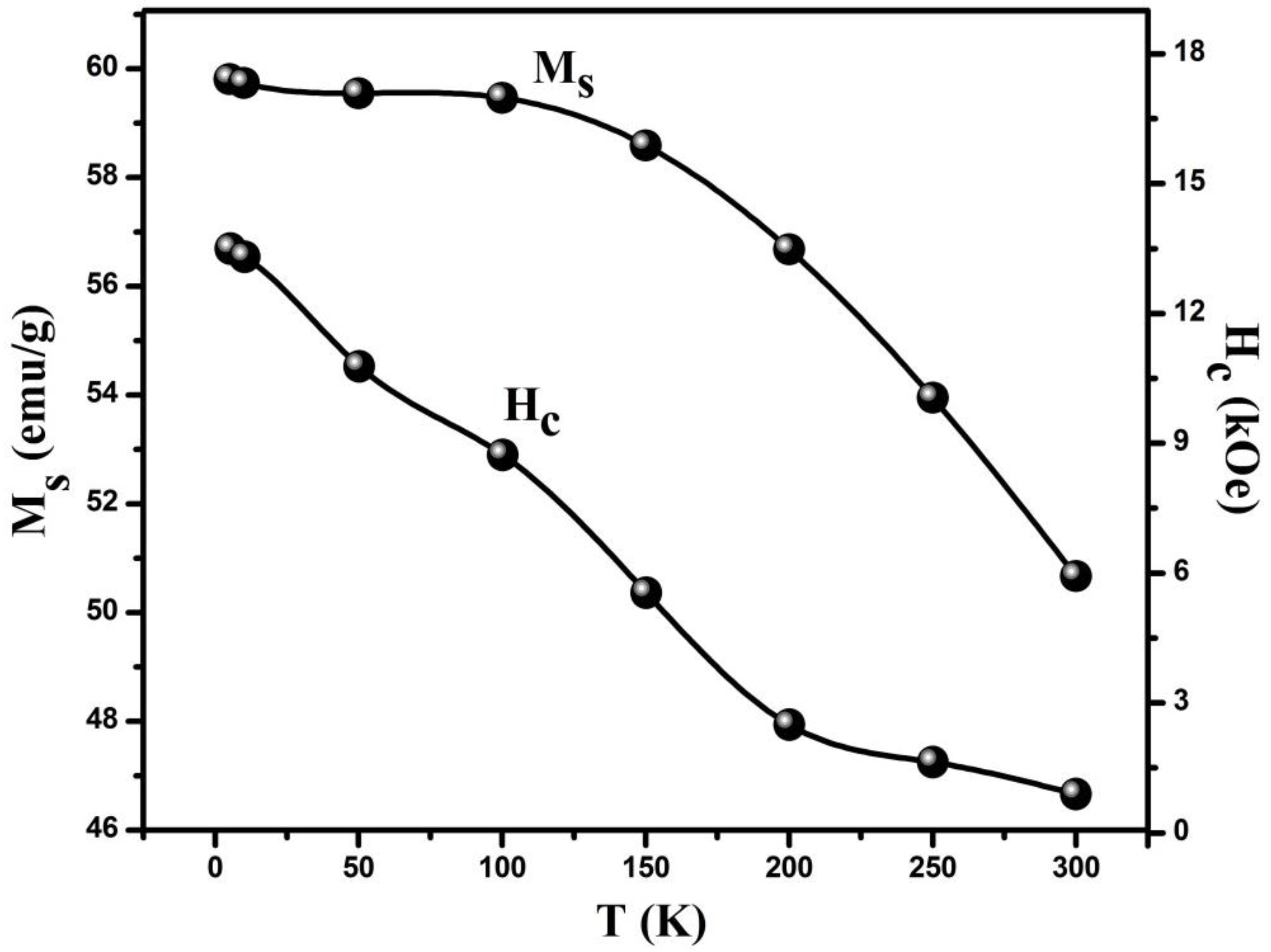
3.4. Magnetic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ikenaga, N.; Ohgaito, Y.; Matsushima, H.; Suzuki, T. Role of water vapor in oxidative decomposition of calcium sulfide. Fuel 2004, 83, 671–677. [Google Scholar]
- Jung, C.W.; Jacobs, P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: Ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging 1995, 13, 661–674. [Google Scholar] [CrossRef]
- Liu, J.P.; Fullernton, E.; Gutfleisch, O.; Sellmyer, D.J. Nanoscale Magnetic Materials and Applications 2009, Volume 7, 1032–1034.
- Brusentsov, N.A.; Gogosov, V.V.; Brusentsova, T.N.; Sergeev, A.V.; Jurchenko, N.Y.; Kuznetsov, A.A.; Kuznetsov, O.A.; Shumakov, L.I. Evaluation of ferromagnetic fluids and suspensions for the site-specific radiofrequency-induced hyperthermia of MX11 sarcoma cells in vitro. J. Magn. Magn. Mater. 2001, 225, 113–117. [Google Scholar] [CrossRef]
- Li, X.; Ren, P.; Zhang, J.; Zhang, L.; Liu, G. Preparation and Magnetic Properties of Mn-Zn Ferrites by the co-precipitation Method. J. Wuhan Uni. Tech. Univ. Technol. Mater. Sci. 2009, 24, 875–878. [Google Scholar] [CrossRef]
- Sangmanee, M.; Maensiri, S. Nanostructures and magnetic properties of cobalt ferrite (CoFe2O4) fabricated by electrospinning. Appl. Phys. A 2009, 97, 167–177. [Google Scholar] [CrossRef]
- Shobaky, G.A.; Turky, A.M.; Mostafa, N.Y.; Mohamed, S.K. Effect of preparation conditions on physicochemical, surface and catalytic properties of cobalt ferrite prepared by coprecipitation. J. Alloy. Compd. 2010, 493, 415–422. [Google Scholar] [CrossRef]
- Cheng, F.X.; Jia, J.T.; Xu, Z.G.; Biao, Z.; Liao, C.S.; Yan, C.H.; Chen, L.Y.; Zhao, H.B. Microstructure, magnetic, and magneto-optical properties of chemical synthesized Co–RE (RE=Ho, Er, Tm, Yb, Lu) ferrite nanocrystalline films. J. Appl. Phys. 1999, 86, 2727–2732. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, F.; Liao, C.; Kuang, J.; Xu, Z.; Chen, L.; Zhao, H.; Liu, Z.; Wang, Y.; Zhu, T.; et al. Sol-gel synthesis, magnetic and magneto-optical properties of CoFe2−xTbxO4 nanocrystalline films. J. Magn. Magn. Mater. 1999, 192, 396–402. [Google Scholar] [CrossRef]
- Kamble, R.B.; Mathe, V.L. Nanocrystalline nickel ferrite thick film as an efficient gas sensor at room temperature. Sens. Actuators B 2008, 131, 205–209. [Google Scholar] [CrossRef]
- Darshane, S.L.; Suryavanshi, S.S.; Mulla, I.S. Nanostructured nickel ferrite: A liquid petroleum gas sensor. Ceram. Int. 2009, 35, 1793–1797. [Google Scholar] [CrossRef]
- Ramana, C.V.; Kolekar, Y.D.; Kamala, B.K.; Sinha, B.; Ghosh, K. Correlation between structural, magnetic, and dielectric properties of manganese substituted cobalt ferrite. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Cibert, J.; Bobo, J.-F.; Lüders, U. Development of new materials for spintronics. C. R. Phys. 2005, 6, 977–996. [Google Scholar] [CrossRef]
- Atsufumi, H.; Hiroaki, S.; Hideto, Y.; Zutic, I.; Seki, T.; Mizukami, S.; Swaminathan, R. Roadmap for Emerging Materials for Spintronic Device Applications. Available online: http://arxiv.org/ftp/arxiv/papers/1509/1509.08997 (accessed on 16 September 2015).
- Wang, L.; Zhou, Q.; Li, F. Ionic disorder and Yaffet-Kittel angle in nanoparticles of ZnFe2O4 prepared by sol-gel method. Phys. Status Solidi B 2004, 241, 377–382. [Google Scholar] [CrossRef]
- Yao, C.; Zang, Q.; Goya, F.G.; Torres, T.; Liu, J.; Wu, H.; Ge, M.; Zeng, Y.; Wang, Y.; Jiang, J.Z. ZnFe2O4 Nanocrystals: Synthesis and Magnetic Properties. J. Phys. Chem. C 2007, 111, 12274–12278. [Google Scholar] [CrossRef]
- Hu, X.; Guan, P.; Yan, X. Hydrothermal synthesis of nano-meter microporous zinc ferrite. China Particuol. 2004, 2, 135–137. [Google Scholar] [CrossRef]
- Kundu, A.; Upadhyay, C.; Verma, H. Magnetic properties of a partially inverted zinc ferrite synthesized by a new coprecipitation technique using urea. Phys. Lett. A 2003, 311, 406–410. [Google Scholar] [CrossRef]
- Nawale, A.B.; Kanhe, N.S.; Patil, K.R.; Bhoraskar, S.V.; Mathe, V.L.; Das, A.K. Magnetic properties of thermal plasma synthesized nanocrystalline nickel ferrite (NiFe2O4). J. Alloy. Compd. 2011, 509, 4404–4413. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, J.; Zhao, J.; Sun, Z.; Wang, Y. Sol-Gel Auto-Combustion Synthesis of Zinc Ferrite for Moderate Temperature Desulfurization. Energy Fuels 2007, 21, 2682–2687. [Google Scholar] [CrossRef]
- Adeela, N.; Maaz, K.; Khan, U.; Karim, S.; Nisar, A.; Ahmad, M.; Ali, G.; Han, X.F.; Duan, J.L.; Liu, J. Influence of manganese substitution on structural and magnetic properties of CoFe2O4 nanoparticles. J. Alloy. Compd. 2015, 635, 533–540. [Google Scholar] [CrossRef]
- Maaz, K.; Karim, S.; Lee, K.J.; Jung, M.H. Effect of Zn substitution on the structural and magnetic properties of Ni–Co ferrites. Mater. Chem. Phys. 2012, 133, 1006–1010. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Phadatare, M.R.; Thorat, N.D.; Joshi, R.S.; Yadav, H.M.; Pawar, S.H. Low temperature combustion synthesis and magnetostructural properties of Co–Mn nanoferrites. J. Magn. Magn. Mater. 2014, 352, 91–98. [Google Scholar] [CrossRef]
- Karimia, Z.; Mohammadifar, Y.; Shokrollahi, H.; Khameneh, S.H.; Yousefi, G.H.; Karimi, L. Magnetic and structural properties of nano sized Dy-doped cobalt ferrite synthesized by co-precipitation. J. Magn. Magn. Mater. 2014, 361, 150–156. [Google Scholar] [CrossRef]
- Rana, S.; Philip, J.; Raj, B. Micelle based synthesis of cobalt ferrite nanoparticles and its characterization using Fourier Transform Infrared Transmission Spectrometry and Thermogravimetry. Mater. Chem. Phys. 2010, 124, 264–269. [Google Scholar] [CrossRef]
- Slatineanu, T.; Iordan, A.R.; Palamaru, M.N.; Caltun, A.F.; Gafton, V.; Leontie, L. Synthesis and characterization of nanocrystalline Zn ferrites substituted with Ni. Mater. Res. Bull. 2011, 46, 1455–1460. [Google Scholar] [CrossRef]
- Samoila, P.; Slatineanu, T.; Postolache, P.; Iordan, A.R.; Palamaru, M.N. The effect of chelating/combustion agent on catalytic activity and magnetic properties of Dy doped Ni–Zn ferrite. Mater. Chem. Phys. 2012, 136, 241–246. [Google Scholar] [CrossRef]
- Trivedi, U.N.; Jani, K.H.; Modi, K.B.; Joshi, H.H. Study of cation distribution in lithium doped nickel ferrite. J. Mater. Sci. Lett. 2000, 19, 1271–1273. [Google Scholar] [CrossRef]
- Amer, M.A.; Meaz, T.M.; Kestawy, M.E.; Ghoneim, A.I. Magnetic and structural studies of trivalent Co-substituted Cd–Mn ferrites. J. Magn. Magn. Mater. 2016, 405, 137–144. [Google Scholar] [CrossRef]
- Park, Y.; Ito, Y.; Imanishi, Y. pH-Controlled gating of a porous glass filter by surface grafting of polyelectrolyte brushes. Chem. Mater. 1997, 9, 2755–2758. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, Y.; Bruening, M.; Bergbreiter, D.; Crooks, R. Inhibition of Electrochemical Reactions at Gold Surfaces by Grafted, Highly Fluorinated, Hyperbranched Polymer Films. Langmuir 1997, 13, 1388–1391. [Google Scholar] [CrossRef]
- Imry, Y.; Ma, S.-K. Random-Field Instability of the Ordered State of Continuous Symmetry. Phys. Rev. Lett. 1975, 35, 1399–1401. [Google Scholar] [CrossRef]
- Shu, S.; Strauser, C.G.; Wollan, W.A. Neutron Diffraction by Paramagnetic and Antiferromagnetic Substances. Phys. Rev. 1951, 83, 333–345. [Google Scholar]
- Ghosh, B.; Kumar, S.; Poddar, A.; Mazumdar, C.; Banerjee, S.; Reddy, V.R.; Gupte, A. Spin glasslike behavior and magnetic enhancement in nanosized Ni–Zn ferrite system. J. Appl. Phys. 2010, 108. [Google Scholar] [CrossRef]
- Rondinone, A.J.; Samia, A.C.S.; Zhang, Z.J. Superparamagnetic Relaxation and Magnetic Anisotropy Energy Distribution in CoFe2O4 Spinel Ferrite Nanocrystallites. J. Phys. Chem. B 1999, 103, 6876–6880. [Google Scholar] [CrossRef]
- Wasilewski, P.J. Magnetic hysteresis in natural materials. Earth Planet. Sci. Lett. 1973, 20, 67–74. [Google Scholar] [CrossRef]
- Gao, Q.; Hong, G.; Ni, J.; Wang, W.; Tang, J.; He, J. Uniaxial anisotropy and ovel magnetic behaviours of CoFe2O4 nanoparticles prepared in a magnetic field. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Heisz, S.; Hilscher, G. The origin of graduated demagnetization curves of NdFeB magnets. J. Magn. Magn. Mater. 1987, 67, 20–28. [Google Scholar] [CrossRef]
- Roberts, A.P.; Cui, Y.; Verosub, K.L. Wasp-waisted hysteresis loops: Mineral magnetic characteristics and discrimination of components in mixed magnetic systems. J. Geophys. Res. 1995, 100, 17909–17924. [Google Scholar] [CrossRef]
- Melikhov, Y.; Snyder, J.E.; Jiles, D.C.; Ring, A.P.; Paulsen, J.A.; Lo, C.C.H.; Dennis, K.W. Temperature dependence of magnetic anisotropy in Mn-substituted cobalt ferrite. J. Appl. Phys. 2006, 99. [Google Scholar] [CrossRef]
- Koseoglu, Y.; Baykal, A.; Gozuak, F.; Kavas, H. Structural and magnetic properties of CoxZn1−xFe2O4 nanocrystals synthesized by microwave method. Polyhedron 2009, 28, 2887–2892. [Google Scholar] [CrossRef]
- Liu, B.; Hu, B.; Zuliang, D. Hydrothermal synthesis and magnetic properties of single-crystalline BiFeO3 nanowires. Chem. Commun. 2011, 47, 8166–8168. [Google Scholar] [CrossRef] [PubMed]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Bertino, M.F. Temperature dependent coercivity and magnetization of nickel ferrite nanoparticles. J. Magn. Magn. Mater. 2010, 322, 2199–2202. [Google Scholar] [CrossRef]
- Mandal, K.; Mitra, S.; Kumar, P.A. Deviation from Bloch T3/2 law in ferrite nanoparticles. Europhys. Lett. 2006, 75, 618–623. [Google Scholar] [CrossRef]
- Toksha, B.G.; Shirsath, S.E.; Mane, M.L.; Patange, S.M.; Jadhav, S.S.; Jadhav, K.M. Autocombustion High-Temperature Synthesis, Structural, and Magnetic Properties of CoCrxFe2–xO4 (0 ≤ x ≤ 1.0). J. Phys. Chem. C 2011, 115, 20905–20912. [Google Scholar] [CrossRef]
- Khan, U.; Adeela, N.; Javed, K.; Riaz, S.; Ali, H.; Iqbal, M.; Han, X.F.; Naseem, S. Influence of cobalt doping on structural and magnetic properties of BiFeO3 nanoparticles. J. Nanopart. Res. 2015, 17, 429–436. [Google Scholar] [CrossRef]
- Bala, T.; Sankar, C.R.; Baidakova, M.; Osipov, V.; Enoki, T.; Joy, P.A.; Prasad, B.L.V.; Sastry, M. Cobalt and magnesium ferrite nanoparticles: Preparation using liquid foams as templates and their magnetic characteristics. Langmuir 2005, 21, 10638–10643. [Google Scholar] [CrossRef] [PubMed]


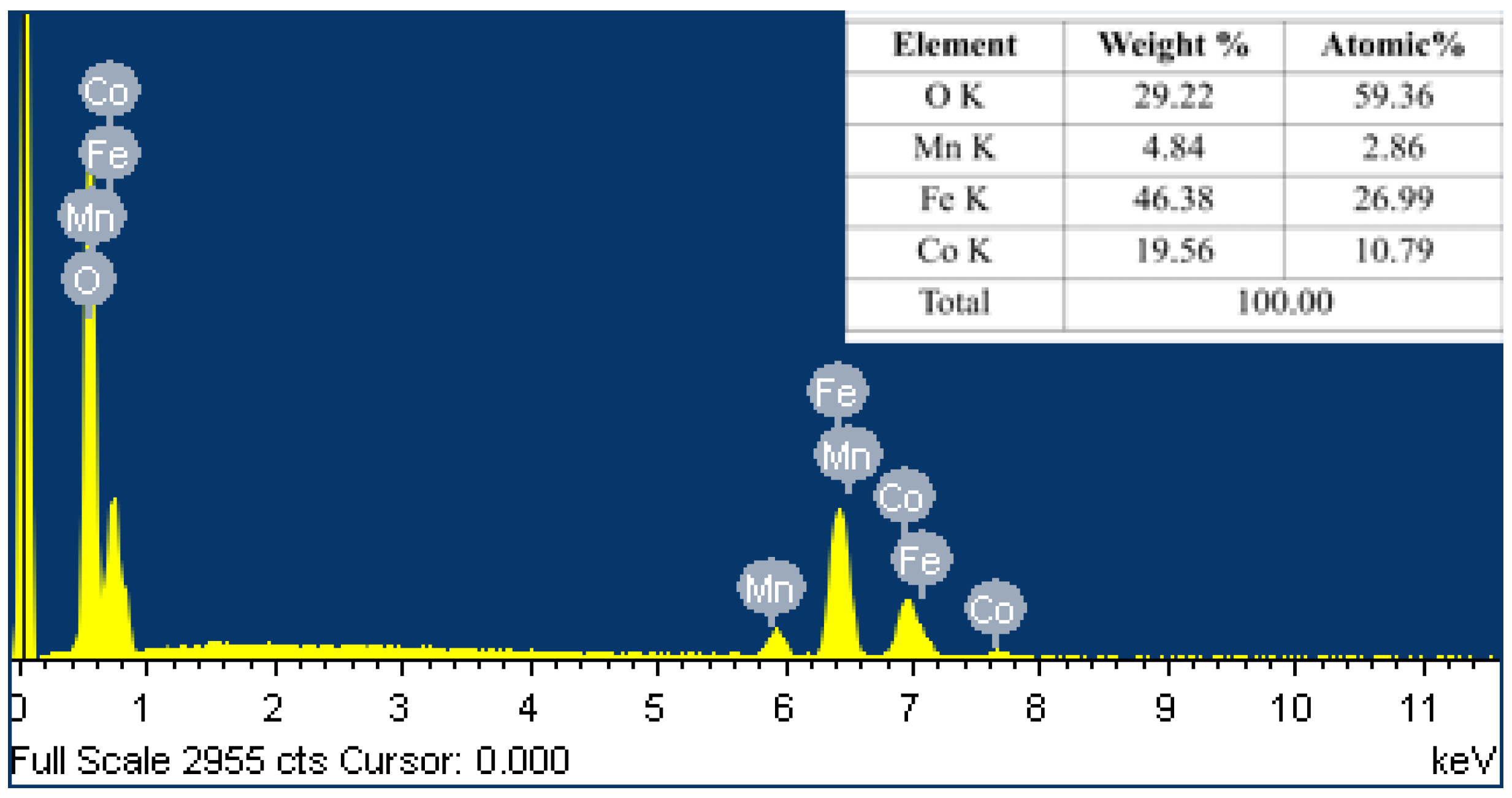


| Parameters | Values |
|---|---|
| Crystallite Size | 14.33 nm |
| Lattice parameter | 8.439 Å |
| Tetrahedral hopping length (LA) | 3.654 Å |
| Octahedral hopping length (LB) | 2.983 Å |
| Tetrahedral bond length (dAx) | 1.914 Å |
| Octahedral bond length (dAx) | 2.060 Å |
| X-ray density | 5.167 g/cm3 |
| Higher vibrational frequency band (ʋ1) | 546.18 cm−1 |
| Lower vibrational frequency band (ʋ2) | 412.58 cm−1 |
| Force constant at A site | 2.16 × 105 dyne/cm2 |
| Force constant at B site | 1.23 × 105 dyne/cm2 |
| Temperature (K) | Hc (Oe) | Ms (emu/g) | nB (×10−2) | K × 103 (erg/Oe) |
|---|---|---|---|---|
| 5 | 13,499.75 | 59.81 | 2.503 | 841.06 |
| 10 | 13,309.22 | 59.74 | 2.500 | 828.22 |
| 50 | 10,777.68 | 59.55 | 2.492 | 668.55 |
| 100 | 8724.59 | 59.47 | 2.489 | 540.47 |
| 150 | 5542.09 | 58.59 | 2.452 | 338.24 |
| 200 | 2490.22 | 56.68 | 2.372 | 147.02 |
| 250 | 1625.93 | 53.95 | 2.258 | 91.26 |
| 300 | 893.41 | 50.67 | 2.120 | 47.15 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nairan, A.; Khan, M.; Khan, U.; Iqbal, M.; Riaz, S.; Naseem, S. Temperature-Dependent Magnetic Response of Antiferromagnetic Doping in Cobalt Ferrite Nanostructures. Nanomaterials 2016, 6, 73. https://doi.org/10.3390/nano6040073
Nairan A, Khan M, Khan U, Iqbal M, Riaz S, Naseem S. Temperature-Dependent Magnetic Response of Antiferromagnetic Doping in Cobalt Ferrite Nanostructures. Nanomaterials. 2016; 6(4):73. https://doi.org/10.3390/nano6040073
Chicago/Turabian StyleNairan, Adeela, Maaz Khan, Usman Khan, Munawar Iqbal, Saira Riaz, and Shahzad Naseem. 2016. "Temperature-Dependent Magnetic Response of Antiferromagnetic Doping in Cobalt Ferrite Nanostructures" Nanomaterials 6, no. 4: 73. https://doi.org/10.3390/nano6040073
APA StyleNairan, A., Khan, M., Khan, U., Iqbal, M., Riaz, S., & Naseem, S. (2016). Temperature-Dependent Magnetic Response of Antiferromagnetic Doping in Cobalt Ferrite Nanostructures. Nanomaterials, 6(4), 73. https://doi.org/10.3390/nano6040073