Receptor-Mediated Drug Delivery Systems Targeting to Glioma
Abstract
:1. Introduction
2. Single Ligand-Modified Targeting Systems

| Target Targeting Site | BCECs | Glioma | Targeting Moiety | Nanocarrier | References |
|---|---|---|---|---|---|
| TfR | √ | √ | Tf | PEGylated nanoscaled GO | [21] |
| Magnetic silica PLGA NPs | [17] | ||||
| OX26 | Immunomicelles | [22] | |||
| T7 | Dendrigraft poly-l-lysine | [23] | |||
| Dendrigraft poly-l-lysine | [24] | ||||
| TfR-lytic hybrid peptide | TfR-lytic hybrid peptide | [25,26] | |||
| LR | √ | √ | Lf | Superparamagnetic iron oxide NPs | [27] |
| LDLR | √ | √ | nLDL | nLDL | [28] |
| Peptide-22 | PEG-PLA NPs | [29] | |||
| LRP | √ | √ | Angiopep-2 | Gold NPs | [30] |
| PEG-PCL NPs | [31] | ||||
| MTf | — | [32] | |||
| FR | √ | √ | Folate | MnO NPs | [33] |
| PEGylated PEI | [34] | ||||
| Insulin Receptor | √ | √ | 83-14 murine monoclonal antibody | PEGylated immunoliposomes | [35] |
| Interleukin-13 Receptor | × | √ | IL-13(IP) | Mesoporous silica NPs | [36] |
| Mesoporous silica-coated graphene nanosheet | [37] | ||||
| Integrin | × | √ | RGD | Nanochain | [38] |
2.1. Cascade-Targeting Systems
2.1.1. TfR-Mediated Targeting Systems
2.1.2. LfR-Mediated Targeting Systems
2.1.3. LDLR-Mediated Targeting Systems
2.1.4. LRP-Mediated Targeting Systems
2.1.5. FR-Mediated Targeting Systems
2.1.6. Other Receptor-Mediated Targeting Systems
2.2. Glioma-Targeting Systems
2.2.1. ILR-Mediated Targeting Systems
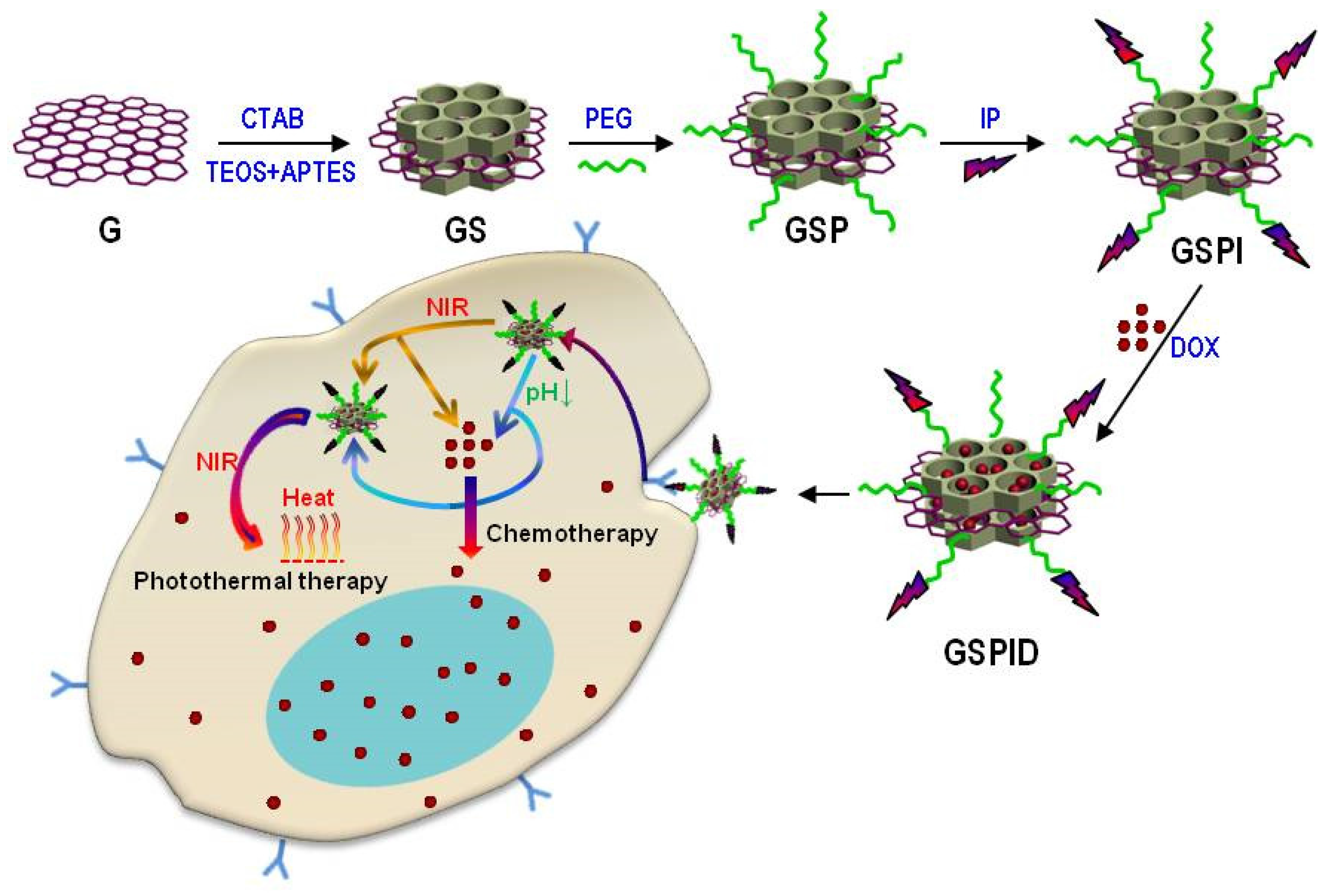
2.2.2. Integrin-Mediated Targeting Systems
3. Dual Ligand-Modified Targeting Systems
3.1. Cascade-Targeting Systems
| Nanocarrier | Targeting Moiety | Targeting Site | Payload | Reference |
|---|---|---|---|---|
| PAMAM dendrimer | Tf | TfR | DOX | [104] |
| WGA | WGA receptor | |||
| PAMAM dendrimer | Tf | TfR | DOX | [103] |
| TAM | ABC transporters | |||
| PEG-PLA NPs | Lf | LfR | PTX | [106] |
| tLyP-1 | Neuropilin-1 | |||
| Liposomes | Angiopep-2 | LRP | VEGF siRNA, Docetaxel | [107] |
| tLyP-1 | Neuropilin-1 | |||
| Liposomes | Tf | TfR | DOX | [108] |
| TAT | — | |||
| PEG-PCL NPs | Angiopep-2 | LRP | DOX | [102] |
| EGFP-EGF1 | Specific tissue factor | |||
| Polymer-lipid hybrid NPs | Folate | FR | PTX | [109] |
| RGD | Integrin | |||
| PEGylated Liposomes | OX26 | TfR | PC27 | [110] |
| CTX | Matrix metalloproteinase-2 |
3.2. Glioma-Targeting Systems
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agnihotri, S.; Burrell, K.E.; Wolf, A.; Jalali, S.; Hawkins, C.; Rutka, J.T.; Zadeh, G. Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch. Immunol. Ther. Exp. 2013, 61, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Paw, I.; Carpenter, R.C.; Watabe, K.; Debinski, W.; Lo, H.W. Mechanisms regulating glioma invasion. Cancer Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Ju, R.J.; Li, X.Y.; Zeng, F.; Shi, J.F.; Liu, L.; Zhang, C.X.; Sun, M.G.; Lou, J.N.; Lu, W.L. Multifunctional targeting daunorubicin plus quinacrine liposomes, modified by wheat germ agglutinin and tamoxifen, for treating brain glioma and glioma stem cells. Oncotarget 2014, 5, 6497–6511. [Google Scholar] [PubMed]
- Cornago, M.; Garcia-Alberich, C.; Blasco-Angulo, N.; Vall-Llaura, N.; Nager, M.; Herreros, J.; Comella, J.X.; Sanchis, D.; Llovera, M. Histone deacetylase inhibitors promote glioma cell death by G2 checkpoint abrogation leading to mitotic catastrophe. Cell Death Dis. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Improving drug delivery to primary and metastatic brain tumors: Strategies to overcome the blood-brain barrier. Clin. Pharmacol. Ther. 2015, 97, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.G.; Gaillard, P.J. Drug targeting to the brain. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.Z.; McDannold, N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood-brain barrier disruption: A safety study. J. Control. Release 2015, 204, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Manich, G.; Cabezon, I.; Del, V.J.; Duran-Vilaregut, J.; Camins, A.; Pallas, M.; Pelegri, C.; Vilaplana, J. Study of the transcytosis of an anti-transferrin receptor antibody with a Fab’ cargo across the blood-brain barrier in mice. Eur. J. Pharm. Sci. 2013, 49, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-brain barrier endogenous transporters as therapeutic targets: A new model for small molecule CNS drug discovery. Expert Opin. Ther. Targets 2015, 19, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.D.; Pan, J.; Connolly, I.D.; Remington, A.; Wilson, C.M.; Grant, G.A. Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg. Focus. 2015, 38. [Google Scholar] [CrossRef] [PubMed]
- Beduneau, A.; Saulnier, P.; Benoit, J.P. Active targeting of brain tumors using nanocarriers. Biomaterials 2007, 28, 4947–4967. [Google Scholar] [CrossRef] [PubMed]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, Q.; Chow, P.K.; Wang, D.; Wang, C.H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xing, L.; Chen, Y.; Xu, Y.; Yang, F.; Zhang, C.; Ping, Q.; Xiao, Y. Lactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol. Pharm. 2014, 11, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Hayavi, S.; Halbert, G.W. Synthetic low-density lipoprotein, a novel biomimetic lipid supplement for serum-free tissue culture. Biotechnol. Prog. 2005, 21, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chiang, C.F.; Chen, L.F.; Liang, P.C.; Hsieh, W.Y.; Lin, W.L. Polymersomes conjugated with des-octanoyl ghrelin and folate as a BBB-penetrating cancer cell-targeting delivery system. Biomaterials 2014, 35, 4066–4081. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shen, H.; Mao, J.; Zhang, L.; Jiang, Z.; Sun, T.; Lan, Q.; Zhang, Z. Transferrin modified graphene oxide for glioma-targeted drug delivery: In vitro and in vivo evaluations. ACS Appl. Mater. Interfaces 2013, 5, 6909–6914. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Liu, S.; Wang, R.; Hu, X.; Xie, Z.; Huang, Y.; Jing, X. Fluorescence-labeled immunomicelles: Preparation, in vivo biodistribution, and ability to cross the blood-brain barrier. Macromol. Biosci. 2012, 12, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, Y.; Huang, R.; Li, J.; Huang, S.; Kuang, Y.; Han, L.; Jiang, C. Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials 2012, 33, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; An, S.; Guo, Y.; Huang, S.; Shao, K.; Liu, Y.; Li, J.; Ma, H.; Jiang, C. T7 peptide-functionalized nanoparticles utilizing RNA interference for glioma dual targeting. Int. J. Pharm. 2013, 454, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, M.; Horibe, T.; Kohno, M.; Kawakami, K. A novel transferrin receptor-targeted hybrid peptide disintegrates cancer cell membrane to induce rapid killing of cancer cells. BMC Cancer 2011, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, M.; Kohno, M.; Horibe, T.; Kawakami, K. Immunogenicity and toxicity of transferrin receptor-targeted hybrid peptide as a potent anticancer agent. Cancer Chemother. Pharmacol. 2013, 71, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhu, Y.; Jiang, W.; Zhou, Q.; Yang, H.; Gu, N.; Zhang, Y.; Xu, H.; Xu, H.; Yang, X. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials 2011, 32, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Gibbs, A.R.; Hunt, C.A.; Budinger, T.F.; Forte, T.M. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J. Control. Release 2007, 124, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, X.; Mei, H.; Wang, Y.; Liao, Z.; Chen, J.; Zhang, Q.; Hu, Y.; Pang, Z.; Jiang, X. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials 2013, 34, 9171–9182. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Sha, X.; Jiang, X.; Zhang, W.; Chen, L.; Fang, X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 2012, 33, 8167–8176. [Google Scholar] [CrossRef] [PubMed]
- Gabathulera, R.; Arthura, G.; Kennarda, M.; Chena, Q.; Tsaia, S.; Yanga, J.; Schoorla, W.; Vitalisb, T.Z.; Jefferies, W.A. Development of a potential protein vector (NeuroTrans) to deliver drugs across the blood-brain barrier. Int. Congr. Ser. 2005, 1277, 171–184. [Google Scholar] [CrossRef]
- Leamon, C.P.; Low, P.S. Folate-mediated targeting: From diagnostics to drug and gene delivery. Drug Discov. Today 2001, 6, 44–51. [Google Scholar] [CrossRef]
- Chen, N.; Shao, C.; Qu, Y.; Li, S.; Gu, W.; Zheng, T.; Ye, L.; Yu, C. Folic acid-conjugated MnO nanoparticles as a T1 contrast agent for magnetic resonance imaging of tiny brain gliomas. ACS Appl. Mater. Interfaces 2014, 6, 19850–19857. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.W.; Gumbleton, M. Endocytosis at the blood-brain barrier: From basic understanding to drug delivery strategies. J. Drug Target. 2006, 14, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Thaci, B.; Brown, C.E.; Binello, E.; Werbaneth, K.; Sampath, P.; Sengupta, S. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro-Oncology 2014, 16, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, W.; Song, W.; Wang, L.; Liu, X.; Jiang, C.; Huang, R. Tumor cell targeted delivery by specific peptide-modified mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 14608–14616. [Google Scholar] [CrossRef]
- Slegerova, J.; Hajek, M.; Rehor, I.; Sedlak, F.; Stursa, J.; Hruby, M.; Cigler, P. Designing the nanobiointerface of fluorescent nanodiamonds: Highly selective targeting of glioma cancer cells. Nanoscale 2015, 7, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, Y.; Zhai, S.; Chen, Q.; Xing, D. Dihydroartemisinin and transferrin dual-dressed nano-graphene oxide for a pH-triggered chemotherapy. Biomaterials 2015, 62, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Sohda, T.; Ueda, S.; Tanaka, T.; Hirano, G.; Yokoyama, K.; Morihara, D.; Aanan, A.; Takeyama, Y.; Irie, M.; et al. Immunohistochemical demonstration of transferrin receptor 1 and 2 in human hepatocellular carcinoma tissue. Hepatogastroenterology 2014, 61, 426–430. [Google Scholar] [PubMed]
- Neves, J.V.; Caldas, C.; Wilson, J.M.; Rodrigues, P.N. Molecular mechanisms of hepcidin regulation in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol. 2011, 31, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Bernabeu, E.; Rodriguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta 2012, 1820, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, L.; Yin, Q.; Zhang, Z.; Feng, L.; Li, Y. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: Synthesis, preparation and in vivo evaluation. J. Control. Release 2012, 159, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.W.; Park, S.C.; Choi, C.; Nam, J.P.; Kim, T.H.; Choi, S.K.; Park, J.K.; Nah, J.W. Anticancer effect of gene/peptide co-delivery system using transferrin-grafted LMWSC. Int. J. Pharm. 2015, 488, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Patil, R.; Portilla-Arias, J.; Ding, H.; Konda, B.; Espinoza, A.; Mongayt, D.; Markman, J.L.; Elramsisy, A.; Phillips, H.W.; et al. Nanobiopolymer for direct targeting and inhibition of EGFR expression in triple negative breast cancer. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Pardridge, W.M. Biotin delivery to brain with a covalent conjugate of avidin and a monoclonal antibody to the transferrin receptor. J. Pharmacol. Exp. Ther. 1992, 263, 897–903. [Google Scholar] [PubMed]
- Paris-Robidas, S.; Emond, V.; Tremblay, C.; Soulet, D.; Calon, F. In vivo labeling of brain capillary endothelial cells after intravenous injection of monoclonal antibodies targeting the transferrin receptor. Mol. Pharmacol. 2011, 80, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Fan, Y.; He, B.; Zheng, N.; Yuan, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; et al. Bionano interactions of MCF-7 breast tumor cells with a transferrin receptor targeted nanoparticle. Mol. Pharm. 2015, 12, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, J.; Huang, S.; Huang, R.; Liu, S.; Hu, X.; Yi, P.; Shan, D.; Wang, X.; Lei, H.; et al. Peptide-conjugated polyamidoamine dendrimer as a nanoscale tumor-targeted T1 magnetic resonance imaging contrast agent. Biomaterials 2011, 32, 2989–2998. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Fan, Y.; Zheng, N.; He, B.; Yuan, L.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials 2013, 34, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.; Mei, L.; Gao, H.; Shi, K.; Chen, J.; Wang, Y.; Zhang, Q.; Yang, Y.; He, Q. Enhanced glioma targeting and penetration by dual-targeting liposome co-modified with T7 and TAT. J. Pharm. Sci. 2014, 103, 3891–3901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, T.; Zhang, W.; Jiang, Y.; Jin, H.; He, H.; Yang, V.C.; Chen, Y.; Huang, Y. A Prodrug-Type, MMP-2-Targeting Nanoprobe for Tumor Detection and Imaging. Theranostics 2015, 5, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, X.; Gong, S.; Feng, L.; Zhong, Y.; Pang, Z. The proton permeability of self-assembled polymersomes and their neuroprotection by enhancing a neuroprotective peptide across the blood-brain barrier after modification with lactoferrin. Nanoscale 2014, 6, 3250–3258. [Google Scholar] [CrossRef] [PubMed]
- Almond, R.J.; Flanagan, B.F.; Antonopoulos, A.; Haslam, S.M.; Dell, A.; Kimber, I.; Dearman, R.J. Differential immunogenicity and allergenicity of native and recombinant human lactoferrins: Role of glycosylation. Eur. J. Immunol. 2013, 43, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Raei, M.; Rajabzadeh, G.; Zibaei, S.; Jafari, S.M.; Sani, A.M. Nano-encapsulation of isolated lactoferrin from camel milk by calcium alginate and evaluation of its release. Int. J. Biol. Macromol. 2015, 79, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Gajbhiye, K.R.; Soni, V. Lactoferrin-appended solid lipid nanoparticles of paclitaxel for effective management of bronchogenic carcinoma. Drug Deliv. 2015, 22, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Lonnerdal, B. Baculovirus expression of mouse lactoferrin receptor and tissue distribution in the mouse. BioMetals 2004, 17, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.J.; Takeuchi, T.; Harada, E. Receptor-mediated transport of lactoferrin into the cerebrospinal fluid via plasma in young calves. J. Vet. Med. Sci. 2003, 65, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Q.; Ke, W.L.; Qu, Y.H.; Zhu, J.H.; Pei, Y.Y.; Jiang, C. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J. Biomed. Sci. 2007, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.H.; Lai, Y.H.; Chiu, T.L.; Chen, Y.Y.; Hu, S.H.; Chen, S.Y. Magnetic core-shell nanocapsules with dual-targeting capabilities and co-delivery of multiple drugs to treat brain gliomas. Adv. Healthc. Mater. 2014, 3, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Liu, Y.; Jiang, C.; Pei, Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials 2008, 29, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaissa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Bertato, M.P.; Oliveira, C.P.; Wajchenberg, B.L.; Lerario, A.C.; Maranhao, R.C. Plasma kinetics of an LDL-like nanoemulsion and lipid transfer to HDL in subjects with glucose intolerance. Clinics 2012, 67, 347–353. [Google Scholar] [CrossRef]
- Huang, C.L.; Hsiao, I.L.; Lin, H.C.; Wang, C.F.; Huang, Y.J.; Chuang, C.Y. Silver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cells. Environ. Res. 2015, 136, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.J.K.J. Low density lipoproteins mediated nanoplatforms for cancer targeting. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Kader, A.; Pater, A. Loading anticancer drugs into HDL as well as LDL has little affect on properties of complexes and enhances cytotoxicity to human carcinoma cells. J. Control. Release 2002, 80, 29–44. [Google Scholar] [CrossRef]
- Chakraborty, S.; Cai, Y.; Tarr, M.A. In vitro oxidative footprinting provides insight into apolipoprotein B-100 structure in low-density lipoprotein. Proteomics 2014, 14, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.C.; Tsang, S.Y.; Lo, E.H.; Fung, K.P. Low density lipoprotein as a targeted carrier for doxorubicin in nude mice bearing human hepatoma HepG2 cells. Life Sci. 2001, 70, 591–601. [Google Scholar] [CrossRef]
- Sarkar, G.; Curran, G.L.; Mahlum, E.; Decklever, T.; Wengenack, T.M.; Blahnik, A.; Hoesley, B.; Lowe, V.J.; Poduslo, J.F.; Jenkins, R.B. A carrier for non-covalent delivery of functional beta-galactosidase and antibodies against amyloid plaques and IgM to the brain. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.J.; Verma, I.M. Targeted delivery of proteins across the blood-brain barrier. Proc. Natl. Acad. Sci. USA 2007, 104, 7594–7599. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, Y.; Currie, J.C.; Poirier, J.; Demeule, M.; Abulrob, A.; Fatehi, D.; Stanimirovic, D.; Sartelet, H.; Castaigne, J.P.; Beliveau, R. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br. J. Cancer 2011, 105, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Sagare, A.P.; Friedman, A.E.; Bedi, G.S.; Holtzman, D.M.; Deane, R.; Zlokovic, B.V. Transport pathways for clearance of human Alzheimer’s amyloid β-peptide and apolipoproteins E and J in the mouse central nervous system. J. Cereb. Blood Flow Metab. 2007, 27, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.A.; Hegg, R.; Freitas, F.R.; Tavares, E.R.; Almeida, C.P.; Baracat, E.C.; Maranhao, R.C. Effect of neoadjuvant chemotherapy on low-density lipoprotein (LDL) receptor and LDL receptor-related protein 1 (LRP-1) receptor in locally advanced breast cancer. Braz. J. Med. Biol. Res. 2012, 45, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.E.; Flaherty, S.L.; Johanson, C.E.; Duncan, J.R.; Silverberg, G.D.; Miller, M.C.; Tavares, R.; Yang, W.; Wu, Q.; Sabo, E.; et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006, 112, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, D.A.; Koning, N.; Schuurman, K.G.; van Strien, M.E.; van Eden, C.G.; Hamann, J.; Huitinga, I. Selective upregulation of scavenger receptors in and around demyelinating areas in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2013, 72, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Kastin, A.J.; Zankel, T.C.; van Kerkhof, P.; Terasaki, T.; Bu, G. Efficient transfer of receptor-associated protein (RAP) across the blood-brain barrier. J. Cell Sci. 2004, 117, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Regina, A.; Che, C.; Poirier, J.; Nguyen, T.; Gabathuler, R.; Castaigne, J.P.; Beliveau, R. Identification and design of peptides as a new drug delivery system for the brain. J. Pharmacol. Exp. Ther. 2008, 324, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Currie, J.C.; Bertrand, Y.; Che, C.; Nguyen, T.; Regina, A.; Gabathuler, R.; Castaigne, J.P.; Beliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res. 2013, 19, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Suryo, R.Y.; Bal, S.; Loh, K.H.; Yu, Y.; Richardson, D.R. Melanotransferrin: Search for a function. Biochim. Biophys. Acta 2012, 1820, 237–243. [Google Scholar]
- Karkan, D.; Pfeifer, C.; Vitalis, T.Z.; Arthur, G.; Ujiie, M.; Chen, Q.; Tsai, S.; Koliatis, G.; Gabathuler, R.; Jefferies, W.A. A unique carrier for delivery of therapeutic compounds beyond the blood-brain barrier. PLoS ONE 2008, 3. [Google Scholar] [CrossRef]
- Cheng, D.; Cao, N.; Chen, J.; Yu, X.; Shuai, X. Multifunctional nanocarrier mediated co-delivery of doxorubicin and siRNA for synergistic enhancement of glioma apoptosis in rat. Biomaterials 2012, 33, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.D.; Choi, S.H.; Kim, D.H.; Lee, H.Y.; Choi, K.C. Self-organized nanoparticles based on chitosan-folic acid and dextran succinate-doxorubicin conjugates for drug targeting. Arch. Pharm. Res. 2014, 37, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chiang, C.F.; Wu, S.K.; Chen, L.F.; Hsieh, W.Y.; Lin, W.L. Targeting microbubbles-carrying TGFβ1 inhibitor combined with ultrasound sonication induce BBB/BTB disruption to enhance nanomedicine treatment for brain tumors. J. Control. Release 2015, 211, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jeong, L.H.; Boado, R.J.; Pardridge, W.M. Receptor-mediated delivery of an antisense gene to human brain cancer cells. J. Gene Med. 2002, 4, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Liu, Y.C. Cardiolipin-incorporated liposomes with surface CRM197 for enhancing neuronal survival against neurotoxicity. Int. J. Pharm. 2014, 473, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Gururangan, S.; Friedman, H.S. Innovations in design and delivery of chemotherapy for brain tumors. Neuroimaging Clin. N. Am. 2002, 12, 583–597. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, W. The blood-brain/tumor barriers: Challenges and chances for malignant gliomas targeted drug delivery. Curr. Pharm. Biotechnol. 2012, 13, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Kioi, M.; Seetharam, S.; Puri, R.K. Targeting IL-13RA2-positive cancer with a novel recombinant immunotoxin composed of a single-chain antibody and mutated Pseudomonas exotoxin. Mol. Cancer Ther. 2008, 7, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Pang, Z.; Yang, X.; Jiang, X. Glioma-homing peptide with a cell-penetrating effect for targeting delivery with enhanced glioma localization, penetration and suppression of glioma growth. J. Control. Release 2013, 172, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Geskin, L.J.; Viragova, S.; Stolz, D.B.; Fuschiotti, P. Interleukin-13 is overexpressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood 2015, 125, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Leland, P.; Kobayashi, H.; Choyke, P.L.; Jagoda, E.M.; Inoue, T.; Joshi, B.H.; Puri, R.K. Analysis of biodistribution of intracranially infused radiolabeled interleukin-13 receptor-targeted immunotoxin IL-13PE by SPECT/CT in an orthotopic mouse model of human glioma. J. Nucl. Med. 2014, 55, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, L.; Wang, Z.; Zhao, Y.; Wu, L.; Fang, X.; Xu, Q.; Xin, H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor ɑ2-mediated endocytosis. Biomaterials 2014, 35, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Yuki, Y.; Kiyono, H.; Shimaoka, M. Structural basis of blocking integrin activation and deactivation for anti-inflammation. J. Biomed. Sci. 2015, 22. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, S.; Ehemann, V.; Schwab, M. Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor and tumor-endothelial cells in vivo. Cancer Res. 2002, 62, 5139–5143. [Google Scholar] [PubMed]
- Peiris, P.M.; Abramowski, A.; Mcginnity, J.; Doolittle, E.; Toy, R.; Gopalakrishnan, R.; Shah, S.; Bauer, L.; Ghaghada, K.B.; Hoimes, C.; et al. Treatment of Invasive Brain Tumors Using a Chain-like Nanoparticle. Cancer Res. 2015, 75, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qian, J.; Cao, S.; Yang, Z.; Pang, Z.; Pan, S.; Fan, L.; Xi, Z.; Jiang, X.; Zhang, Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials 2012, 33, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Huile, G.; Shuaiqi, P.; Zhi, Y.; Shijie, C.; Chen, C.; Xinguo, J.; Shun, S.; Zhiqing, P.; Yu, H. A cascade targeting strategy for brain neuroglial cells employing nanoparticles modified with angiopep-2 peptide and EGFP-EGF1 protein. Biomaterials 2011, 32, 8669–8675. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, H.; Jia, X.; Lu, W.L.; Lou, J.; Wei, Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials 2012, 33, 3899–3908. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; Jia, X.R.; Du, J.; Ying, X.; Lu, W.L.; Lou, J.N.; Wei, Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.; Mei, L.; Gao, H.; Cai, W.; Zhu, P.; Shi, K.; Chen, J.; Wang, Y.; Gao, F.; He, Q. Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals. Mol. Pharm. 2014, 11, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Jiang, M.; Liu, Z.; Gu, G.; Hu, Q.; Kang, T.; Song, Q.; Yao, L.; Li, W.; Gao, X.; et al. Co-administration of dual-targeting nanoparticles with penetration enhancement peptide for antiglioblastoma therapy. Mol. Pharm. 2014, 11, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Li, J.Q.; Wang, Z.Z.; Dong, D.W.; Qi, X.R. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials 2014, 35, 5226–5239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ma, C.; Bai, E.; Yang, K.; Xu, R. Transferrin and cell-penetrating peptide dual-functioned liposome for targeted drug delivery to glioma. Int. J. Clin. Exp. Med. 2015, 8, 1658–1668. [Google Scholar] [PubMed]
- Agrawal, U.; Chashoo, G.; Sharma, P.R.; Kumar, A.; Saxena, A.K.; Vyas, S.P. Tailored polymer-lipid hybrid nanoparticles for the delivery of drug conjugate: Dual strategy for brain targeting. Colloids Surf. B 2015, 126, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.J.; He, L.; Qiu, S.W.; Li, Y.; Liao, Y.J.; Li, X.P.; Xie, D.; Peng, Y. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol. Cancer 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xiong, Y.; Zhang, S.; Yang, Z.; Cao, S.; Jiang, X. RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol. Pharm. 2014, 11, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Meng, Y.; Li, C.; Qian, M.; Huang, R. Receptor-Mediated Drug Delivery Systems Targeting to Glioma. Nanomaterials 2016, 6, 3. https://doi.org/10.3390/nano6010003
Wang S, Meng Y, Li C, Qian M, Huang R. Receptor-Mediated Drug Delivery Systems Targeting to Glioma. Nanomaterials. 2016; 6(1):3. https://doi.org/10.3390/nano6010003
Chicago/Turabian StyleWang, Shanshan, Ying Meng, Chengyi Li, Min Qian, and Rongqin Huang. 2016. "Receptor-Mediated Drug Delivery Systems Targeting to Glioma" Nanomaterials 6, no. 1: 3. https://doi.org/10.3390/nano6010003
APA StyleWang, S., Meng, Y., Li, C., Qian, M., & Huang, R. (2016). Receptor-Mediated Drug Delivery Systems Targeting to Glioma. Nanomaterials, 6(1), 3. https://doi.org/10.3390/nano6010003







