Suitability of EGCG as a Means of Stabilizing a Porcine Osteochondral Xenograft
Abstract
:1. Introduction
2. Results
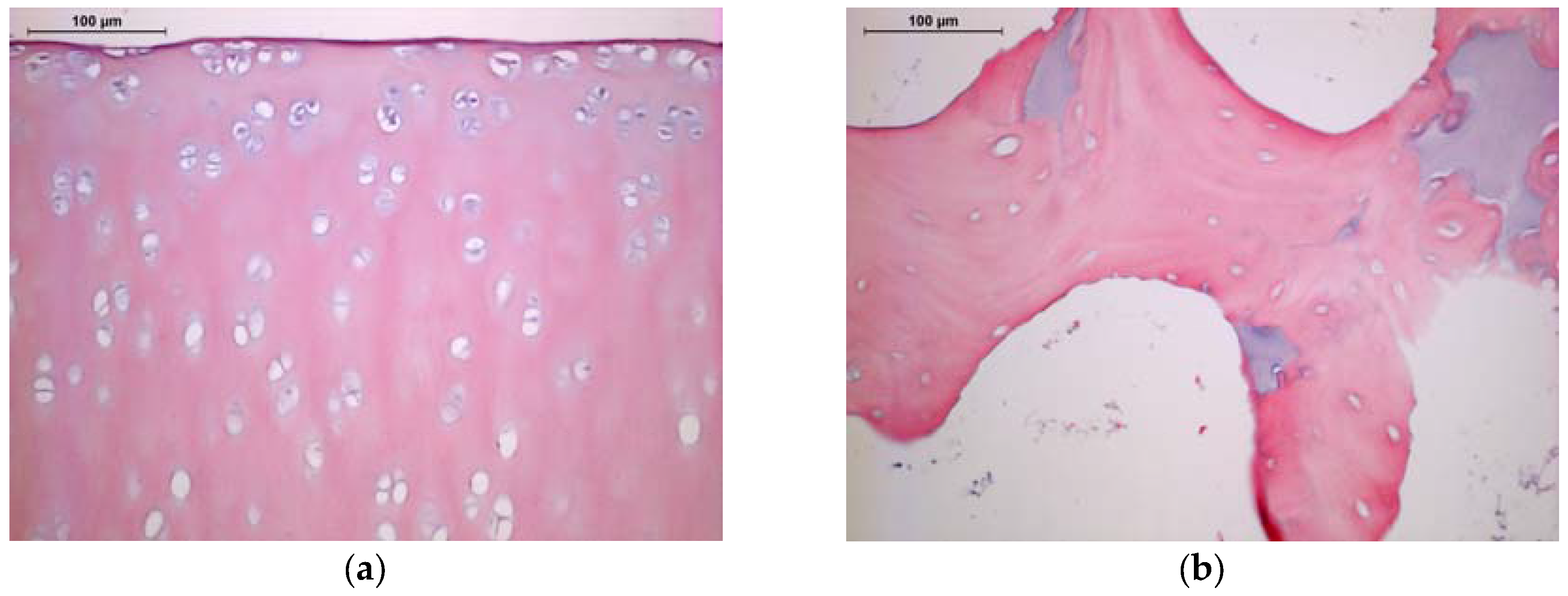
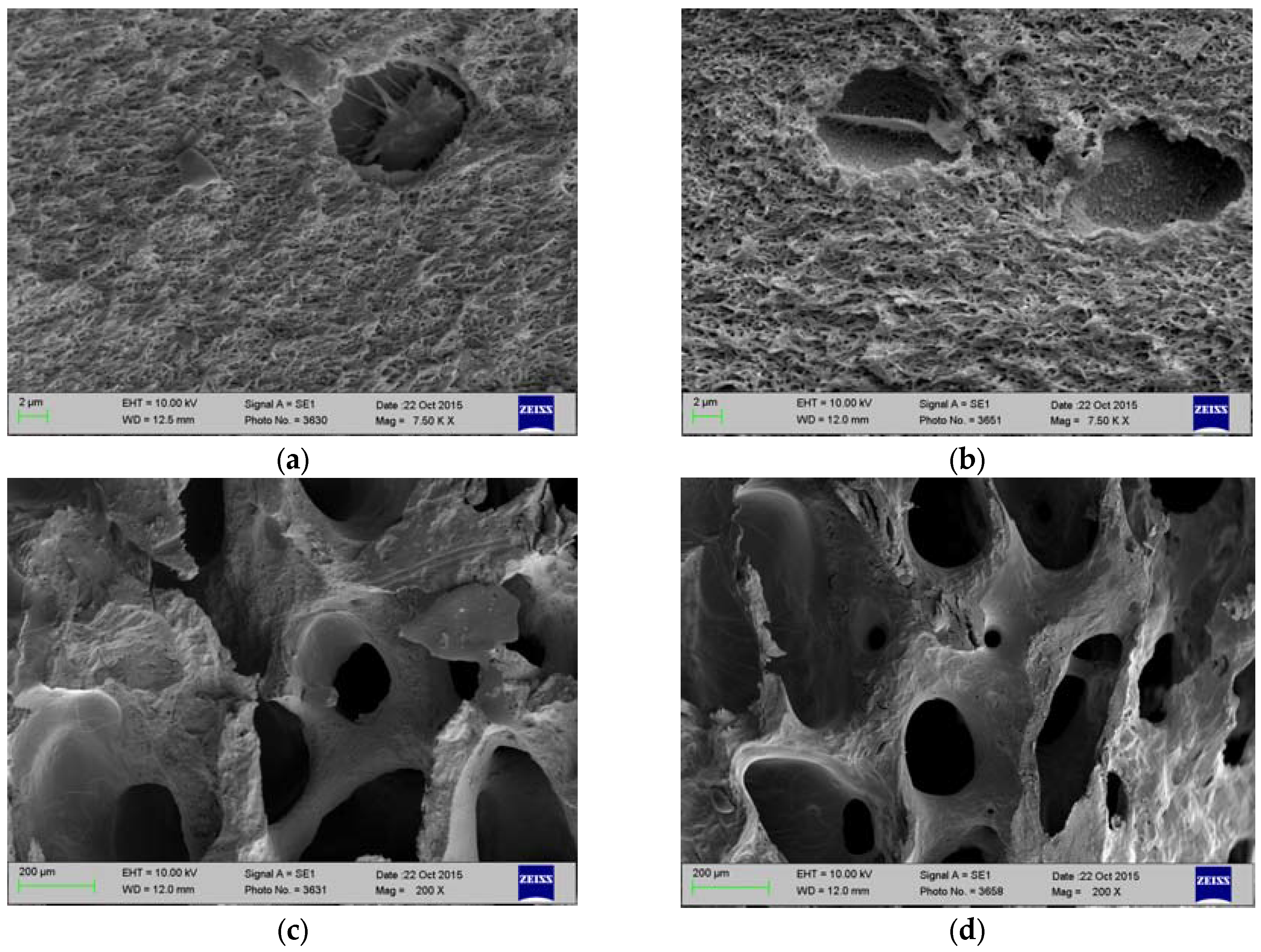
2.1. Histology and SEM
2.2. Degree of Crosslinking
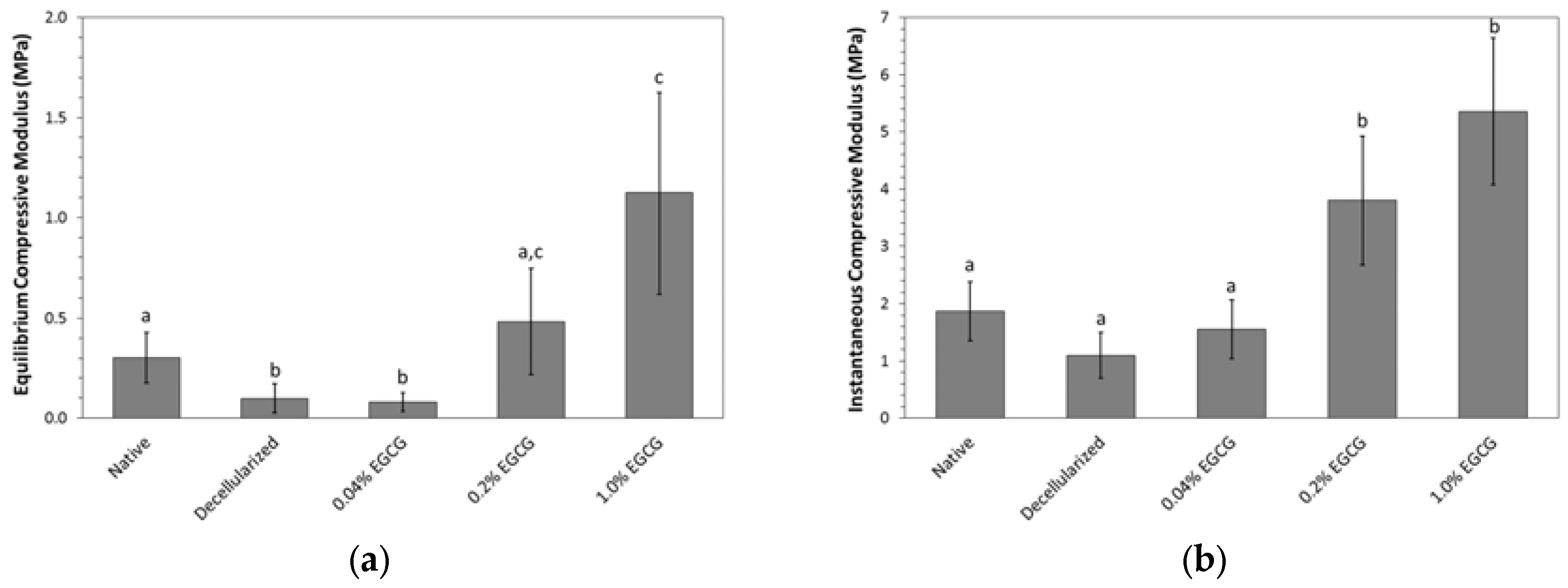
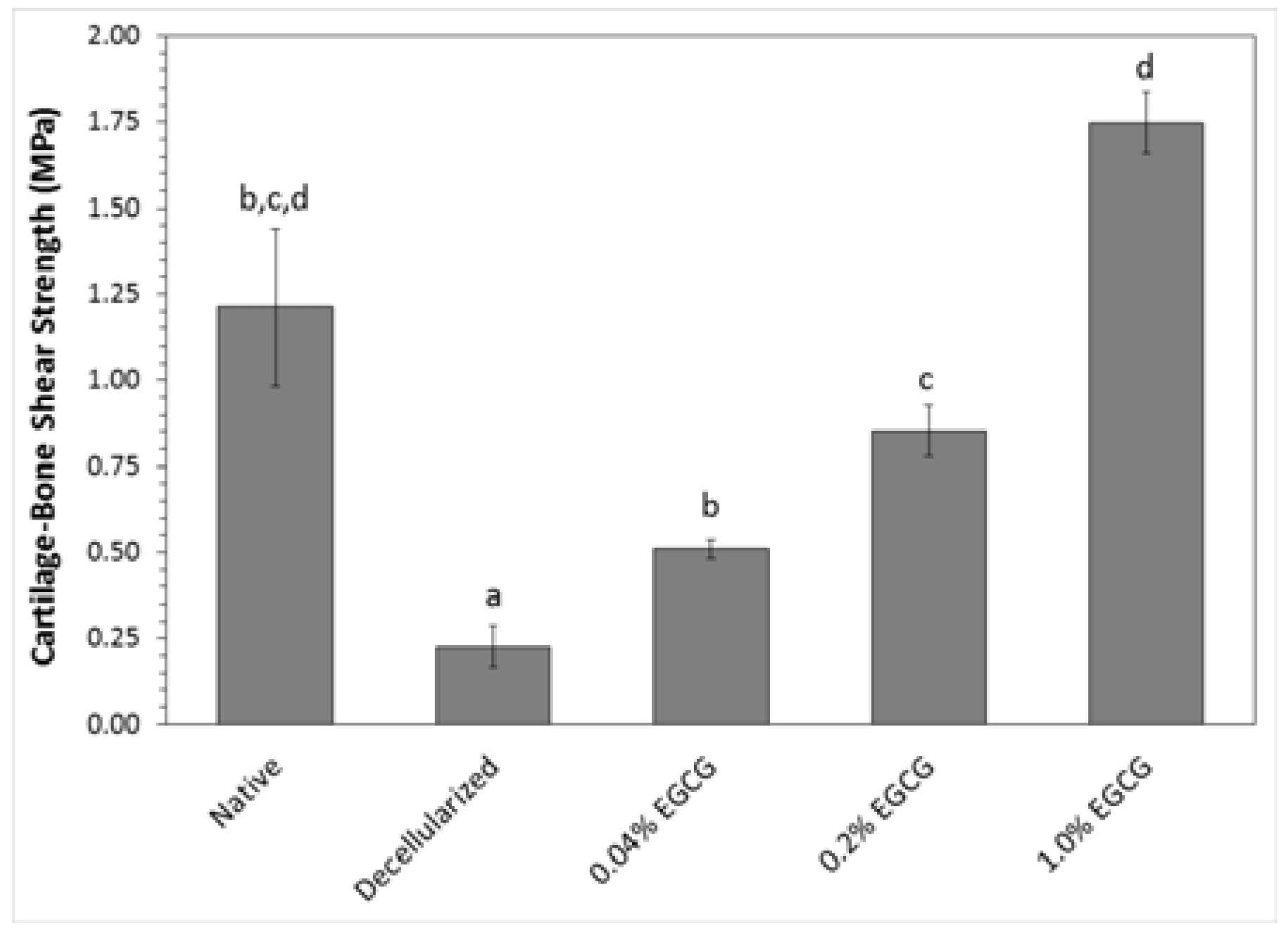
2.3. Mechanical Properties
2.3.1. Cartilage Resistance to Compression
2.3.2. Cartilage-Bone Interface Shear Strength
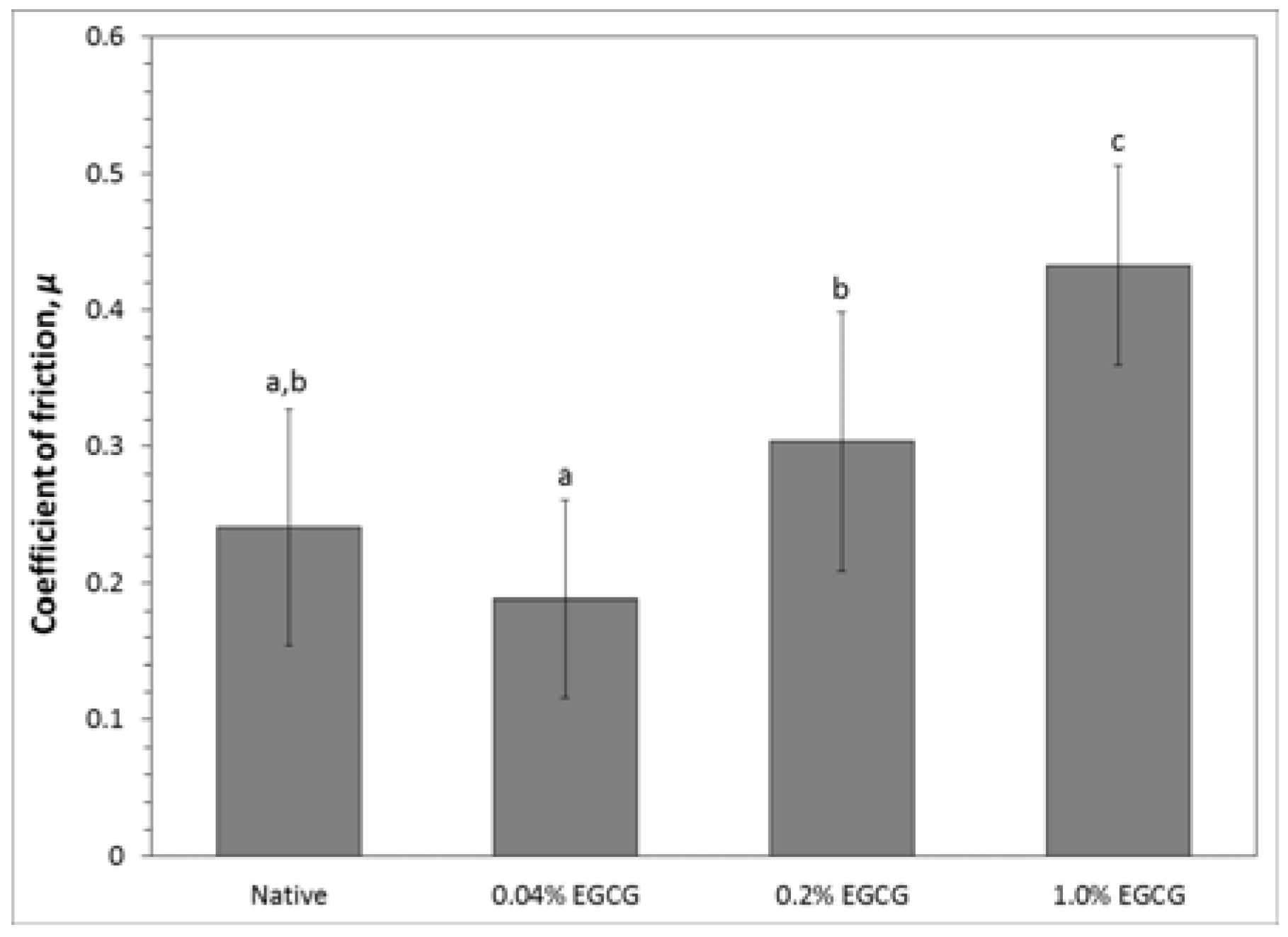
2.3.3. Cartilage Coefficient of Friction
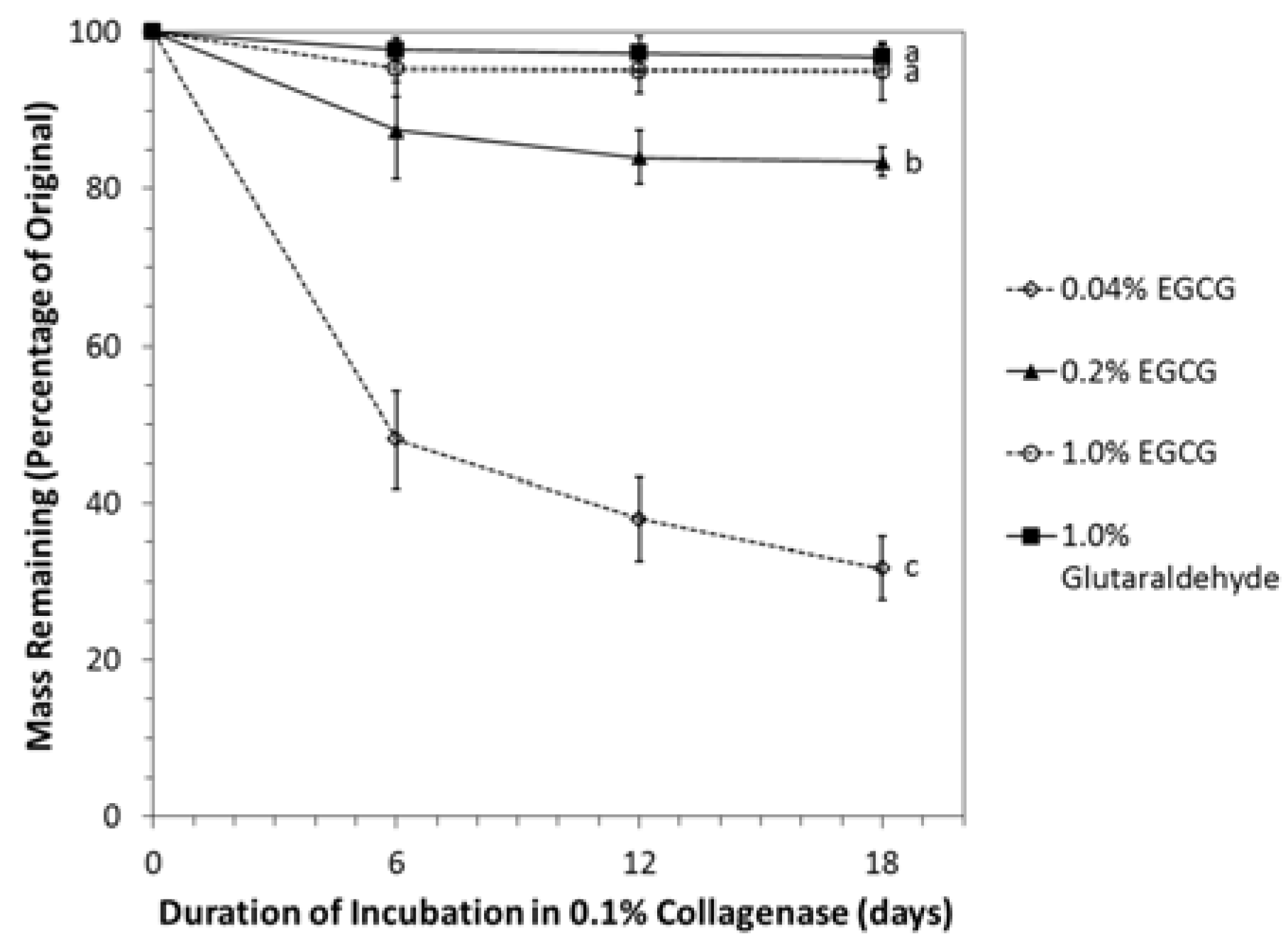
2.4. Resistance of Crosslinked Cartilage to Degradation by Collagenase
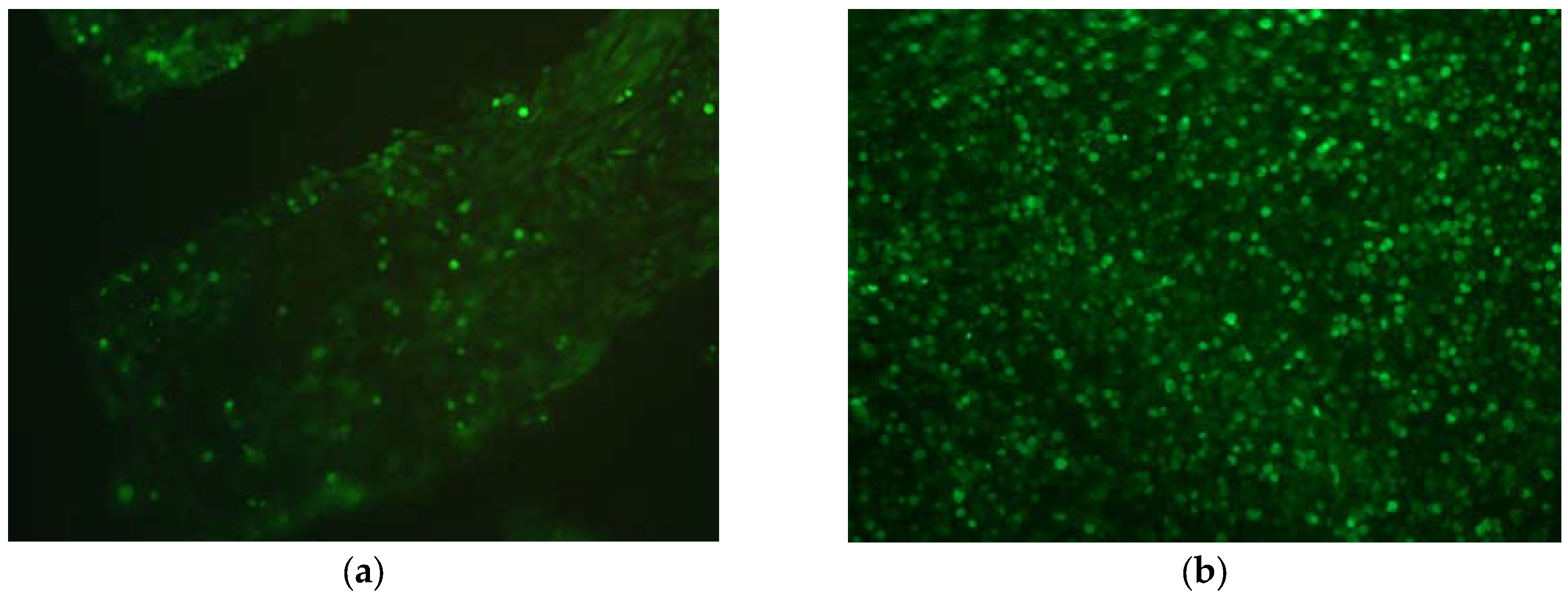
2.5. Cytotoxicity and Biocompatibility
3. Discussion
4. Materials and Methods
4.1. Decellularization and Crosslinking
4.2. Characterization of Antigen Removal by Histology and SEM
4.3. Determination of Degree of Crosslinking by Ninhydrin Assay
4.4. Evaluation of Mechanical Properties
4.4.1. Unconfined Compression of Cartilage
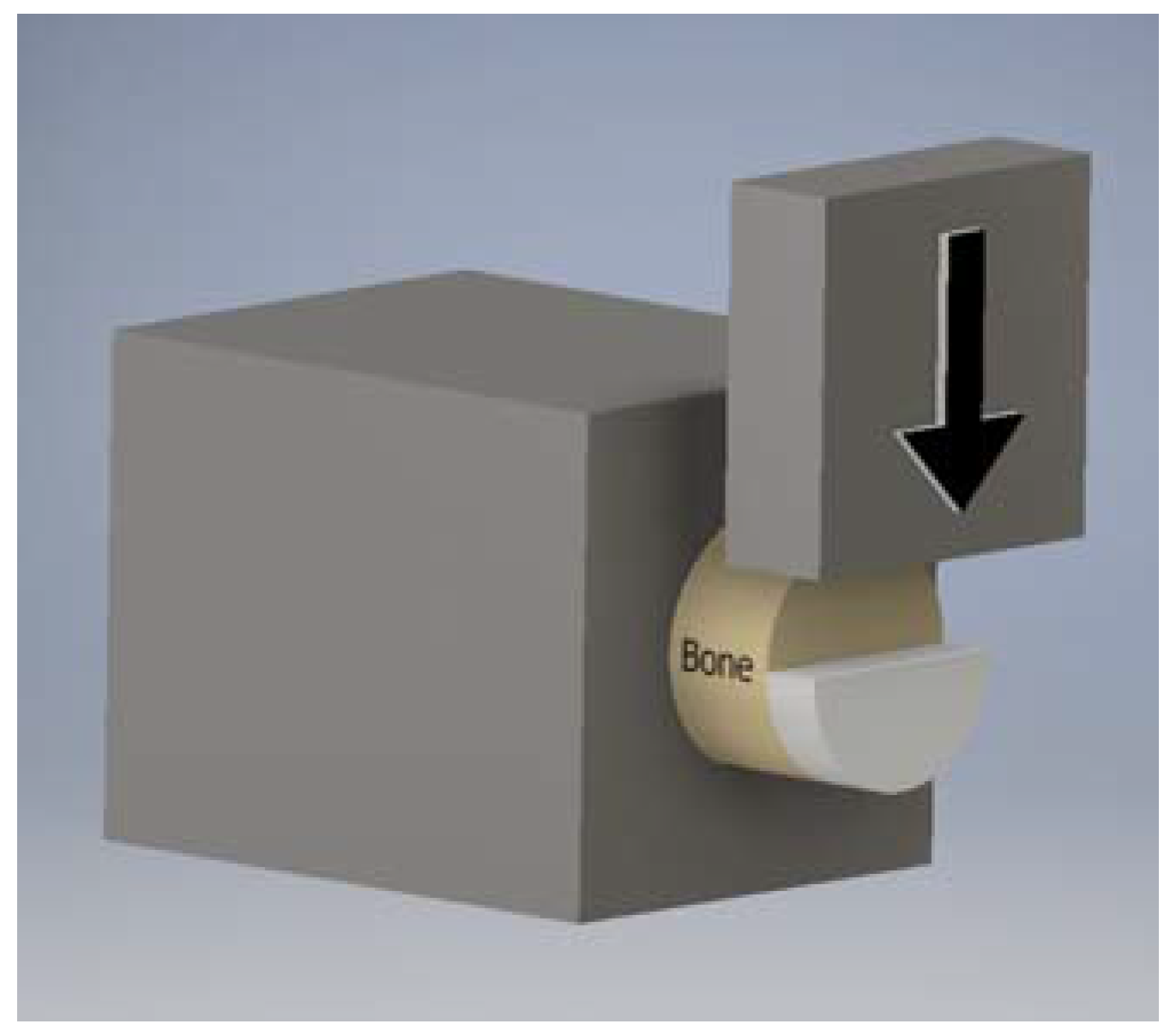
4.4.2. Cartilage-Bone Interface Shear
4.4.3. Cartilage Coefficient of Friction
4.5. Resistance to Degradation by Collagenase
4.6. In Vitro Cytotoxicity and Biocompatibility
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heir, S.; Nerhus, T.K.; Røtterud, J.H.; Løken, S.; Ekeland, A.; Engebretsen, L.; Arøen, A. A comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am. J. Sports Med. 2010, 38, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuelle, C.; Nuelle, J.; Cook, J.; Stannard, J. Patient factors, donor age, and graft storage duration affect osteochondral allograft outcomes in knees with or without comorbidities. J. Knee Surg. 2017, 30, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.; Gracitelli, G.; Shah, N.; Chang, E.Y.; Gomoll, A. High failure rate of a decellularized osteochondral allograft for the treatment of cartilage lesions. Am. J. Sports Med. 2016, 44, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Vilsack, T.; Clark, C. United States Summary and State Data; United States Department of Agriculture: Washington, DC, USA, 2014; Volume 1, Part 51.
- Pellegata, A.F.; Bottagisio, M.; Boschetti, F.; Ferroni, M.; Bortolin, M.; Drago, L.; Lovati, A.B. Terminal sterilization of equine-derived decellularized tendons for clinical use. Mater. Sci. Eng. C 2017, 75, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.; Londono, R.; Turner, N.; Badylak, S. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Kheir, E.; Stapleton, T.; Shaw, D.; Jin, Z.; Fisher, J.; Ingham, E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J. Biomed. Mater. Res. A 2011, 99, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Fermor, H.; Russell, S.; Williams, S.; Fisher, J.; Ingham, E. Development and characterization of a decellularised bovine osteochondral biomaterial for cartilage repair. J. Mater. Sci. Mater. Med. 2015, 26, 186. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.; Cooley, A.; Liao, J.; Prabhu, R.; Elder, S. Comparison of natural crosslinking agents for stabilization of xenogenic articular cartilage. J. Orthop. Res. 2016, 34, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.; Pinheiro, A.; Young, C.; Smith, P.; Wright, E. Evaluation of genipin for stabilization of decellularized porcine cartilage. J. Orthop. Res. 2017, 35, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Akens, M.; von Rechenberg, B.; Bittmann, P.; Nadler, D.; Zlinsky, K.; Auer, J. Long term in-vivo studies of a photo-oxidized bovine osteochondral transplant in sheep. BMC Musculoskelet. Disord. 2001, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Oungoulian, S.R.; Hehir, K.E.; Zhu, K.; Willis, C.E.; Marinescu, A.G.; Merali, N.; Ahmad, C.S.; Hung, C.T.; Ateshian, G.A. Effect of glutaraldehyde fixation on the frictional response of immature bovine articular cartilage explants. J. Biomech. 2014, 47, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Elder, B.; Mohan, A.; Athanasiou, K. Beneficial effects of exogenous crosslinking agents on self-assembled tissue engineered cartilage construct biomechanical properties. J. Mech. Med. Biol. 2011, 11, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Slusarewicz, P.; Hedman, T. Thermal analysis reveals differential effects of various crosslinkers on bovine annulus fibrosis. J. Orthop. Res. 2011, 29, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Englert, C.; Blunk, T.; Müller, R.; von Glasser, S.S.; Baumer, J.; Fierlbeck, J.; Heid, I.M.; Nerlich, M.; Hammer, J. Bonding of articular cartilage using a combination of biochemical degradation and surface cross-linking. Arthritis Res. Ther. 2007, 9, R47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGann, M.; Bonitsky, C.; Jackson, M.; Ovaert, T.; Trippel, S.; Wagner, D. Genipin crosslinking of cartilage enhances resistance to biochemical degradation and mechanical wear. J. Orthop. Res. 2015, 33, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Von Rechenberg, B.; Akens, M.K.; Nadler, D.; Bittmann, P.; Zlinszky, K.; Kästner, S.B.; Auer, J.A. Mosaicplasty with photooxidized, mushroom shaped, bovine, osteochondral xenografts in experimental sheep. Vet. Comp. Orthop. Traumatol. 2006, 19, 147–156. [Google Scholar] [PubMed]
- Natarajan, V.; Madhan, B.; Tiku, M. Intra-articular injections of polyphenols protect articular cartilage from inflammation-induced degradation: Suggesting a potential role in cartilage therapeutics. PLoS ONE 2015, 10, e0127165. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Grenier, D. Regulation of matrix metalloproteinase secretion by green tea catechins in a three-dimensional co-culture model of macrophages and gingival fibroblasts. Arch. Oral Biol. 2017, 75, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yan, J.; Luo, L.; Ma, M.; Zhu, H. Preparation and characterization of (−)-epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 45521. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.; Haqqi, T. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappa. Arthritis Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, S.; Singh, A.; Chourasia, M.; Ahmed, S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1β signaling in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharmacol. 2017, 329, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, H.; Hwang, Y.; Rosa, V.; Yu, M.; Min, K. Effects of epigallocatechin gallate, an antibacterial cross-linking agent, on proliferation and differentiation of human dental pulp cells cultured in collagen scaffolds. J. Endod. 2017, 43, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.; Nair, B. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Covington, A.; Hancock, R. Structure—Activity relationships in the hydrophobic interactions of polyphenols with cellulose and collagen. Biopolymers 2003, 70, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.M.; Aguiar, T.R.; Phansalkar, R.; McAlpine, J.B.; Napolitano, J.G.; Chen, S.N.; Araújo, L.S.; Pauli, G.F.; Bedran-Russo, A. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014, 10, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Kumar, B.; Shanmugan, G.; Madhan, B.; Mandal, A. Molecular level insights on collagen-polyphenols interaction using spin-relaxation and saturation transfer difference NMR. J. Phys. Chem. B 2015, 119, 14076–14085. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Xiang, L.; Wu, Y.; Wei, X.; Qu, Y.; Man, Y. Evaluation of epigallocatechin-3-gallate (EGCG) cross-linked collagen membranes and concerns on osteoblasts. Mater. Sci. Eng. C 2016, 67, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Sono, R.; Sofiqul, I.; Yiu, C.; Nakamura, H.; Otsuki, M.; Takatsuka, T.; Tagami, J. In vitro evaluation of plant-derived agents to preserve dentin collagen. Dent. Mater. 2013, 29, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, Y.O.; Ryu, J.G.; Oh, H.J.; Son, H.J.; Lee, S.H.; Kwon, J.E.; Kim, E.K.; Park, M.K.; Park, S.H. Epigallocatechin-3-gallate ameliorates autoimmune arthritis by reciprocal regulation of T helper-17 regulatory T cells and inhibition of osteoclastogenesis by inhibiting STAT3 signaling. J. Leukoc. Biol. 2016, 100, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Joyce, E.; Sacks, M. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials 2008, 29, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Partington, L.; Mordan, N.J.; Mason, C.; Knowles, J.C.; Kim, H.W.; Lowdell, M.W.; Birchall, M.A.; Wall, I.B. Biochemical changes caused by decellularization may compromise mechanical integrity of tracheal scaffolds. Acta Biomater. 2013, 9, 5251–5261. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Afara, I.; Tehrani, A.; Oloyede, A. Effect of decellularization on the load-bearing characteristics of articular cartilage matrix. Tissue Eng. Regen. Med. 2015, 12, 294–305. [Google Scholar] [CrossRef]
- Gunning, G.; Murphy, B. The effects of decellularization and cross-linking techniques on the fatigue life and calcification of mitral valve chordae tendineae. J. Mech. Behav. Biomed. Mater. 2016, 57, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, X.; Wu, C.; Chang, J. Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds. Regen. Biomater. 2014, 1, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, B.; Chang, J. Preparation of decellularized vascular matrix by co-crosslinking of procyanidins and glutaraldehyde. Biomed. Mater. Eng. 2015, 26, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Manji, R.; Lee, W.; Cooper, D. Xenograft bioprosthetic heart valves: Past, present and future. Int. J. Surg. 2015, 23, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Graneist, C.; Emmrich, F.; Till, H.; Metzger, R.; Aupperle, H.; Schierle, K.; Sack, U.; Boldt, A. Xenogenic esophagus scaffolds fixed with several agents: Comparative in vivo study of rejection and inflammation. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tsai, C.C.; Liang, H.C.; Sung, H.W. Reconstruction of the right ventricular outflow tract with a bovine jugular vein fixed with a naturally occurring crosslinking agent (genipin) in a canine model. J. Thorac. Cardiovasc. Surg. 2001, 122, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.C.; Chang, Y.; Hsu, C.K.; Lee, M.H.; Sung, H.W. Effect of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials 2004, 25, 3541–3552. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of differently cross-linked collagen membranes: An experimental study in the rat. Clin. Oral Implants Res. 2005, 16, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bhrany, A.D.; Lien, C.J.; Beckstead, B.L.; Futran, N.D.; Muni, N.H.; Giachelli, C.M.; Ratner, B.D. Crosslinking of an oesophagus acellular matrix tissue scaffold. J. Tissue Eng. Regen. Med. 2008, 2, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.; Hwang, Y.; Choi, Y.; Cho, H.; Suh, H. Development of collagenase-resistant collagen and its interaction with adult human dermal fibroblasts. Biomaterials 2003, 24, 5099–5113. [Google Scholar] [CrossRef]
- Han, B.; Jaurequi, J.; Tang, B.W.; Nimni, M. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. J. Biomed. Mater. Res. A 2003, 65, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Jia, J.; Guo, Y.; Lu, Y.; Zhu, P. Preparation and characterization of IPN hydrogels composed on chitosan and gelatin crosslinked by genipin. Carbohydr. Polym. 2014, 99, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Elder, S.; Cooley, A.; Borazjani, A.; Sowell, B.; To, H.; Tran, S. Production of hyaline-like cartilage by bone marrow mesenchymal stem cells in a self-assembly model. Tissue Eng. Part A 2009, 15, 3025–3036. [Google Scholar] [CrossRef] [PubMed]

| EGCG Concentration (mM) | Percent Cytotoxicity |
|---|---|
| 0.005 | 0.9 |
| 0.05 | 2.0 |
| 0.5 | 10.9 |
| 5 | 51.6 |
| 50 | 54.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elder, S.; Clune, J.; Walker, J.; Gloth, P. Suitability of EGCG as a Means of Stabilizing a Porcine Osteochondral Xenograft. J. Funct. Biomater. 2017, 8, 43. https://doi.org/10.3390/jfb8040043
Elder S, Clune J, Walker J, Gloth P. Suitability of EGCG as a Means of Stabilizing a Porcine Osteochondral Xenograft. Journal of Functional Biomaterials. 2017; 8(4):43. https://doi.org/10.3390/jfb8040043
Chicago/Turabian StyleElder, Steven, John Clune, Jaylyn Walker, and Paul Gloth. 2017. "Suitability of EGCG as a Means of Stabilizing a Porcine Osteochondral Xenograft" Journal of Functional Biomaterials 8, no. 4: 43. https://doi.org/10.3390/jfb8040043




